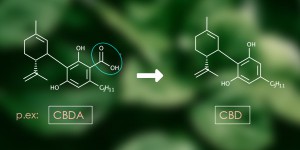
This article will set out two types of secondary metabolites that are biosynthesised by the plant Cannabis sativa L. and probably produce synergy with the effects cannabinoids.
It is being observed that cannabinoids are not the only active substances in the Cannabis plant. Certain studies showed that there were differences between the effects produced by pure cannabinoids and the ones caused by the plant, although cannabinoids are administered in equal doses in both cases. These observations point to the existence of other active substances in the Cannabis plant, which have an intrinsic pharmacological action and/or can modify the pharmacological action of cannabinoids. Two groups of active substances have been identified presently; terpenes and flavonoids, both of which appear in sufficient concentrations to have pharmacological activity. From a scientific perspective, neither which kind of specific compounds are capable of producing synergy with cannabinoids, nor how they are formed, have been proven. Both terpenes and flavonoids are under the increasing attention of the scientific and medical community due to their proven pharmacological actions. In the following paragraphs, we will try to show the current state of the studies about the biological and synergistic activity between these active substances and the cannabinoids.
Terpenes
Terpenes are volatile organic compounds formed by the union of hydrocarbon of 5 carbon atoms, known as isoprene. The smallest and most volatile compounds are monoterpenes, which are biosynthesised by the union of two isoprene molecules. The biggest and least volatile are biosynthesised by the union of three or more isoprene molecules. The sesquiterpenes are next in the chain, which are formed by the union of three isoprene molecules. Terpenes are secondary metabolites, which provide the plant with its organoleptic characteristics (aroma and flavour) and that constitutes most of the essential oil produced by aromatic plants.
Terpenes and cannabinoids share their biosynthetic pathways and, in fact, cannabinoids are terpeno-fenolic compounds. In the Cannabis plant, terpenes also share the biosynthesis and accumulation spaces. Thus, both types of compounds are biosynthesised in the glandular trichomes of leaves and flowers and are accumulated in large proportions in the exuded resin. In any case, it seems that certain non-capitular glandular trichomes, which are more abundant in leaves surface, are specialised in synthesising terpenes. It has been shown that the ratio between monoterpenes and sesquiterpenes in leaves and flowers is rather different. This is due to the dominance of sessile trichomes in leaves, which are more specialised in synthesising terpenes, while capitate trichomes are more abundant in flowers and are specialised in the synthesis of monoterpenes and cannabinoids. The proportion of terpenes in the plant is normally less than 1%, potentially achieving up to 10% of the resin composition.
Terpenes have different functions in plants. The two main ones are the protection against insects and herbivorous animals, as well as protection against high temperatures. Plants react by producing terpenes in the areas affected through the action of insects and herbivorous animals, which act as bitter compounds that repel them or even as pesticides in some cases. Monoterpenes, which are more volatile, dominate in inflorescences to repel insects. Sesquiterpenes, which are more bitter, are more abundant on leaves acting against herbivorous animals. Some terpenes can act as a decoy in some plants, attracting either pollinating insects or predatory ones that feed on herbivorous insects, which are beneficial for the plant. As plants sense a temperature rise, they begin synthesising more terpenes and under high temperatures during night or day, more terpenes are released. Terpenes evaporate at high temperatures, producing airflows that cool the plant and lessen transpiration, preventing the plant from drainage. In the Cannabis plant, terpenes are exuded in the resin and confer it with the sticky and viscous quality that will get some insects trapped and immobilised, thus, acting as a protection against insects and high temperatures. Hence, it is easy to observe that Cannabis plants smell stronger during the first morning hours than during the warmest part of the day as a large amount of terpenes evaporate. This is the reason why it is recommended harvesting the mature plants during the first morning hours, in order to get the maximum production of essential oil.
Cannabis essential oil is mainly formed by a high proportion of monoterpenes and a variable proportion of sesquiterpene. Such proportions, together with the extraction performance, will be mainly affected by the degree of drying that Cannabis achieves when processed for the extraction of the essential oil. In fact, the extraction performance of the essential oil by steam distillation of the fresh plant is lower than 1%, with a composition of 80-90% in monoterpenes and 10-20% in sesquiterpenes. However, it will be around 0.1% in the dried plant and its composition will be lower in monoterpenes, where it can reach 50% in sesquiterpenes, due to the fact that monoterpenes are very volatile and evaporate quickly during the plant drying process. Usually, the essential oil obtained from industrial hemp, which contains many leaves and it is normally processed dried, is chiefly formed by sesquiterpenes. Some sesquiterpenes remain in the plant even after a 15 minute decarboxilation treatment at 120oC. This is the case for cariofileno, which has a moist soil aroma characteristic of baked or cooked Cannabis. Likewise, the evaporation of monoterpenes during the drying process is responsible for the transformation of the aroma from the fresh plant to a well-dried one, although the change in taste comes from the degradation of chlorophylls. Thus, fresh plants have minty, citric, fruity, etc. aromas that soften when dried.
Nevertheless, terpenes are not just responsible of the aroma, but they also have an important biological and therapeutic activity. It has been scientifically shown that the essential oils of plants have therapeutic properties and are the pharmacological base of aromatherapy. These oils and pure terpenes can also be used as flavourings in the food industry, as they are non-toxic compounds. The therapeutic properties will specifically depend on the terpene in question.
The most abundant terpenes in the Cannabis plant that form most of its essential oil are the monoterpenes myrcene, pinene, limonene, linalool, eucalyptol and sesquiterpene caryophyllene. The variation in the ratio between these terpenes is what produces the wide range of aromas that can be found in the Cannabis plant. It has been recently discovered that they can also take part in the varied pharmacologic effects caused by Cannabis, as well as generate synergy with cannabinoids.
Myrcene
Myrcene, or β-myrcene, is a lineal monoterpene carbohydrate and is the main component of the essential oil of wild thyme, comprising 40% of its overall composition. It is found at high concentrations in other plants such as hop, mango and limoncillo, among others. Myrcene acts as an anti-inflammatory interfering in the prostaglandins' metabolic pathway. Myrcene is the sedative active ingredient of the hop, which is used in herbalism and in natural therapies to help with sleeping disorders.
Studies on laboratory animals have shown myrcene's sedative, hypnotic, analgesic and muscle relaxant properties. Its mechanism of action has not been totally unveiled yet, but it could be that it has adrenergic and/or opiod effects, as the analgesic effect is blocked by an antagonistic opioid (naloxone). It has also been shown that the myrcene alters the blood-brain barrier, favouring the penetration of cannabinoids in the brain and increasing the effects.
In a recent study, it was shown that analysing the composition of terpenes in indica varieties against sativa varieties, a greater presence of myrceno was found in indica varieties; up to 60%-80% of their composition. It has been accepted that indica varieties are more relaxing and sedative than sativa varieties. Bringing together all the evidence, we can speculate that the effect of myrceno combined with THC can be highly physical and hypnotic, which is common in indica varieties.
Pinene

Pinene is the common name of two isomer bicyclic monoterpenoids, α-pinene, β-pinene, which are main components of the pine resin and of other conifers, which gives it the name, although it is also the terpene most widely distributed in nature. In fact, it is not only found in the plant kingdom, as the two compounds are part of the chemical communication system of insects and also act as insect repellent.
These components have significant antibiotic effects, even against antibiotic resistant pathogens. Another therapeutic activities attributed to them is that of anti-inflammatories, blocking the inflammatory signal of prostagladins in a similar way to myrcene. They also act as bronchodilator in humans when they are inhaled in low concentrations. This effect could produce a larger absorption of cannabinoids when smoking or when vaporizing Cannabis with a product rich in alpha and beta pineno, which would increase plasma concentrations and, subsequently, the cannabinoids effect.
A-pinene is an acetylcholinesterase inhibitor that may be beneficial for memory and may reduce the negative THC effects on it, although this is no more than a mere assumption at this point. A-pineno has also served as biosynthetic base for the ligands of the cannabinoid receptor CB2. Pineno seems to be quite balanced within the different Cannabis varieties representing around the 10% of the terpenes group and not exceeding 15-20%.
Limonene
Limonene is a cyclic carbohydrate and a main component of the essential oil of lemons and other citrus fruits, which is where its name comes from. It is also the second most widely distributed terpene in nature and it is an intermediate product in other terpenes' biosynthesis. In contrast with pinene, limonene is not found in insects, yet it still has some repellent and insecticide effects. It is widely used in the food and pharmaceutical industries as flavouring. Recent researche has been carried out to look at its usefulness in formulations of dermal patches, to improve the transdermal absorption of other active substances.
Limonene is used in cosmetics and household cleaning product industries as a fragrance and as a biodegradable, organic and environmentally friendly solvent. It is quickly absorbed by inhalation or by the skin and it is metabolised quickly, however there are indications it can accumulate in fatty tissues, such as brain tissue. Limonene is not toxic, nor does it cause skin irritation, yet some of its products, which are oxidised by contact with air, provoke skin and mucous irritation. This lead to 3% of people exposed to high doses for a long period of time, such as the workers of the paint industry, suffering from dermatitis. Nonetheless, limomene has therapeutic effects in certain diseases and some antiseptic properties, mainly against the bacteria responsible for acne.
Studies on laboratory animals suggest that limonene has anxiolytic effects, causing a rise of serotonin and dopamine neurotransmitters in the brain. It has been shown that the dispersal of limonene in the environment has produced a decrease in the depressive symptoms of hospital patients in addition to a strong immunostimulation. Linonene also procuces apoptosis, also called cell death, in breast cancer cells. Its effectiveness is being tested in clinical trials. In adition to this, the use of limomeno against gastroesophageal reflux has been patented.
Linalool
Linalool is a lineal monoterpene alcohol resulting from the main substances of the essential oil of lavender, but it is also found in many other plants. It is widely used as fragrance in cleaning and hygiene products, as an intermediate product in the chemical industry and as insecticide against flies and cockroaches, however it is not useful as an insect repellent. The essential oil of lavender eases skin burns and can even reduce the morphine intake needed, when inhaled by patients with post-operative treatment. These effects are attributed to linalool for being the main component of the essential oil of lavender, as after its ingestion, other substances for example the monoterpene linalyl acetate, hydrolyses into linalool. Linalool in itself has shown to have anxiolytic effects on a comparable level to local anaesthetics such as lidocaine or menthol. It also demonstrates analgesic effects in laboratory animals when mediated by adenosine A2A and glutamate receptors, as well as sedative effects by inhalation.
In addition to these effects, linalool has anti-seizure properties that inhibit glutamatergic activity and is also able to decrease the release of neurotransmitters of the neurons under glutamate stimulation. In this way, we could argue that the sedative, anxiolytic and anti-seizure effects have their mechanism of action based on the modulation of the glutamate and GABA neurotransmitters, similarly to the way the cannabinoides act. Thus, a Cannabis plant with both THC and linalool will probably produce a significant sedative and analgesic effect, due to the synergy between the two compounds. However, a Cannabis plant with CBD and/or THCV and/or CBDV and linalool will probably produce a synergistic effect as an anti-seizure medication, which would be useful in cases of epilepsy, even as a preventive measure.
Eucalyptol

Eucalyptol, also known as 1,8-cineol, is a monoterpene ester that makes up almost the totality of the essential oil of eucalyptus, from which it gets its name, but it is also widely distributed in the plant kingdom. It acts as an insect repellent and insecticide, although it is produced by certain orchids to attract bees. Eucalyptol is used as food additive to add flavour. Products containing eucalyptol need to have less than 0,002% of it, as the intake of greater amounts can affect the central nervous system (CNS) and might even be psychotropic. Eucalyptol is widely used in the cosmetic and chemical industries, but it is still classified as a toxin that might have a negative influence on reproduction. Some researches have shown certain clinical efficacy of eucalyptol for treating asthma and sinusitis, as well as being an anti-inflammatory and a local analgesic.
Furthermore, it has been shown to have immunosuppressive and in vitro anti-leukaemia properties. In the aforementioned study about terpenes profiles in different varieties of Cannabis, it was found that eucalyptol, carene, phellandrene and terpinolene are terpenes found almost exclusively in sativa varieties. Eucalyptol, carene and felandrene are found in proportions close to 5%, whereas terpinolene was around 20% of the total in sativa varieties, while they will not go over 1% in indica varieties. Eucalyptol is the only one of these compounds that has been shown to be active in the CNS, which is almost unique to sativa varieties and that such varieties have a euphoriant effect different from indica varieties. From this we can hypothesise that the synergy between THC and eucalyptol is what makes a difference regarding the qualitative difference of the activating effect of sativa varieties. That being said, the myrcene could also be responsible for the hypnotic effect of indica varieties.
Caryophyllene
Caryophyllene is what we call the mixture of three compounds: α-caryophyllene or humulene, β-caryophyllene, which is the main component of the essential oil of black pepper, and caryophyllene oxide, a result of the oxidation of both melissa and eucalyptus. All of them are bicyclic sesquiterpenic carbohydrate and are present in all Cannabis varieties. In fact, caryophyllene oxide is the signal detected by sniffer dogs trained to find Cannabis. We have to bear in mind that it is one of the less volatile terpenes and that, as mentioned earlier, it resists the process of decarboxylation, thus becoming the terpene most easily found in Cannabis extracts. In the plant kingdom, β-caryophyllene plays an evolutive survivalism role by increasing its release and biosynthesis in plants parasitised by herbivorous insects, so it will attract other predatory insects to reduce the damage produced by herbivores. Caryophyllene oxide takes part in the defence system of the plants, functioning as an insecticide and an antifungal. It should be noted that both caryophyillene and CBC join in the defence against fungi attacks. Moreover, caryophyllene oxide has shown a clinic effectiveness against certain cases of fungal infection. B-caryophyllene, has anti-inflammatory properties and operates at two levels, one is the blocking of the prostaglandins' inflammatory pathway, as happens with myrcene and pinene, and the other is as CB2 cannabinoid receptor agonist.
This last mode of action makes β-caryophyllene the first non-cannabinoid molecule with cannabinomimetic functioning, which is also authorised for human consumption and thus open to a wide therapeutic applicability. Its anti-inflammatory and analgesic effects, as well as its effectiveness in the treatment of atypical dermatitis in animal models has been proven, although not yet in humans. Due to its effects on the prostaglandins inflammatory pathway, caryophyllene also has anticoagulant properties and unexpected gastric protection effects. Gastric ulcers are a secondary effect of certain anti-inflammatory prostaglandins antagonists, which limit their therapeutic effectiveness, however, caryophyllene does not only have this secondary effect, but it can also act as protection against their appearance. Gathering all this evidence, we may predict that Cannabis containing both CBD and caryophyllene will have significant anti-inflammatory and analgesic properties acting on prostaglandins and cannabinoid receptors.