The word cannabinoids refers to every chemical substance, regardless its origin or structure, that joins the cannabinoid receptors of the body and brain and that have similar effects to those produced by the plant Cannabis Sativa L. We know it is a large and varied group of substances that can be classified in several ways, but the most useful way to understand the cannabinoid diversity is the following:
Phytocannabinoids
Phytocannabinoids make reference to the kinds of compounds characterised by 21 carbon atoms which only show in nature in the plant Cannabis Sativa L. Around 70 phytocannabinoids have already been found, including their acidic and neutral forms, their analogous and other transformation products. The plant is just able to synthesise the phytocannabinoids directly in their non-psychoactive forms. Therefore, the main phytocannabinoids present in fresh plant material are Δ9-THCA, CBDA, CBGA y CBCA. However, the carboxyl group is not very stable and it is easily lost as CO2 under the influence of heat or light, which causes the transformation in the active neutral forms. The acidic phytocannabinoids suffer partial decarboxylation in the drying and curing process of buds; subsequently, acidic phytocannabinoids and some of their active neutral forms (Δ9-THC, CBD, CBG y CBC) are mainly found in the plant dry material. A large drying process of the plant material would generate the reduction of acidic phytocannabinoids and the increase of the neutral ones. When the plant is smoked or cooked every acidic cannabinoid suffers decarboxylation in its neutral form due to the influence of heat.
The method normally used in the decarboxylation of small quantities of Cannabis plant material (i.e. 20 grams) consists of placing it in an oven at 120 ºC for a minimum period of 20 minutes. Cooking the Cannabis in butter or oil will also initiate the process for as long as necessary. It is interesting that the most studied phytocannabinoid, Δ9-THC, in its neutral form is the main one responsible for the psychoactive effects caused by Cannabis intake, while it does not show psychoactive activity in its acidic form Δ9-THCA.
Endocannabinoids
Endocannabinoids are produced by almost every organism in the animal kingdom. They are natural endogenous ligands produced by human and animal organisms that join the cannabinoid receptors. Both endocannabinoids and cannabinoid receptors form the endocannabinoid system, which is involved in a large variety of physiological processes, such as the control of the neurotransmitters release, the pain perception and the cardiovascular, gastrointestinal and liver functions. The two main endocannabinoids found are the anandamide (N-arachidonoylethanolamine or ANA) and 2-arachidonoylglycerol (2-AG). Endocannabinoids are the molecules that act as natural key for the main cannabinoid receptors CB1 and CB2 and cause their activation and subsequent action. CB1 is mainly located in the central nervous system and it is responsible for the effects mediated by neuronal processes and psychoactive 'secondary' effects. CB2 is mainly located in the immune system and it is responsible for the immunomodulatory effects. CB2 receptors have been recently discovered in the central nervous system, the microglial cells and they seem to be in certain neurons as well. However, it remains a quite controversial and debated issue.
Synthetic cannabinoids
The main difference between phytocannabinoids, endocannabinoids and synthetic cannabinoids is that the latter are fully synthetic and created in the laboratory. An example of it would be dronabinol (Δ9-THC synthetic), which is the active compound of MARINOL®, a medicine that comes in capsules and has been consumed in the US since 1985 to prevent nausea, vomiting, loss of appetite and loss of weight. Another example would be nabilone, that is the active substance of CESAMET®, a medicine approved for the nausea and vomiting control caused by cancer chemotherapy. Both medicinal products have been approved for these purposes in the US, United Kingdom, Switzerland, Canada and Spain. More recently, some selective cannabinoids for CB1 receptor, such as JHW-018 y JHW-073, have been used as psychoactive ingredients in smart drugs marketed as imitations of Cannabis effects. One of the names used for these drugs is “Spice”. There is not much information about the effects of synthetic cannabinoids in humans, although some of them have already shown to cause more distress and panic than phytocannabinoids. Synthetic cannabinoids have been designed as research tools for cannabinoid scientific studies, however, they have never shown to be reliable for human consumption in clinical testing. In theory, they should have never left the laboratory where they where designed and synthesised.
What part of the plant are phytocannabinoids produced in?
It has been largely accepted that phytocannabinoids are mainly or fully synthesised and stored in small structures called glandular trichomes. Trichomes are present in most of the aerial surfaces of the plant. These structures together with cannabinoids are also found in most of terpenes (monoterpenes and sesquiterpenes), which provide each species with a different aroma, depending on their number and combination. This is the reason why it can be said that trichomes are the most interesting part of Cannabis for pharmacognosy experts.
Cannabis researchers speak of two types of non-glandular trichome (simple unicellular trichomes and natural killer trichomes) that have not been associated with terpenoid biosynthesis. Three types of glandular trichome have been found in female Cannabis plants: bulbous trichome, capitate-sessile trichome and capitate-stalked trichome. It has been shown that male plants have a fourth type of glandular trichome, the glandular trichome of the anthers which only has been found in the anthers.
Even though trichomes can be found in any male and female plant, its highest phytocannabinoid concentration (speaking in % of dry plant material) can be found in female inflorescence's bracts reaching 20% and 25%. Phytocannabinoids are more abundant in capitate-stalked trichome. This kind of trichome appears during the flowering period and forms the thickest cover in pistilated flowers' bracts. A high concentration of capitate-stalked trichome can also be found in the small leaves that go with flowers. Phytocannabinoids are less abundant in plant foliage leaves and stems, while they are quite rare or non-existent in the roots. There are not qualitative dissimilitudes within phytocannabinoids between the different parts of the plant, but there are quantitative ones. The role of phytocannabinoids in plants is unclear. The most plausible hypothesis is that they offer defensive properties against biotic stress (insects, bacteria and fungi) and abiotic stress (drying and ultraviolet radiation) of the plant.
How are phytocannabinoids produced in the plant?
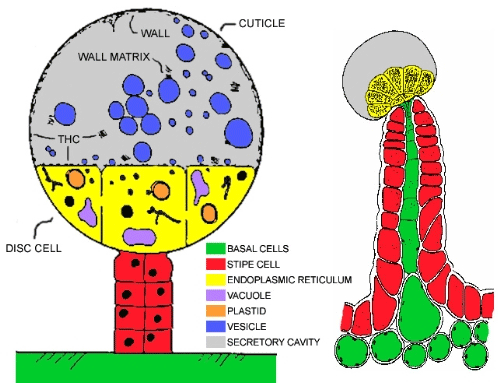
Neither the pathway nor the location of phytocannabinoid biosynthesis are fully known, however, some authors suggest that they are synthesised in specialised disc cells (Picture 1) that appear in glandular trichomes. They are subsequently accumulated in the adjacent secretory cavity and finally emitted as resins, or their synthases are directly secreted in the secretory cavity.
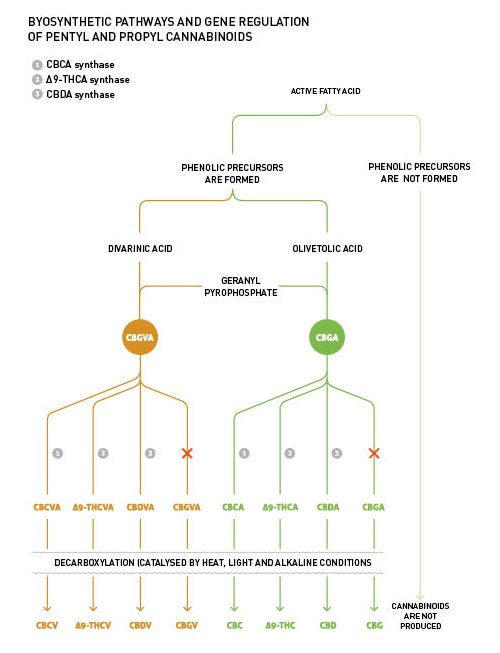
An important structural variation of phytocannabinoids is found in the lateral alkyl chain. In fact, the alky group in the most common phytocannabinoid, Δ9-tetrahidrocannabinol (Δ9-THC), is a pentyl, while it is a propyl in its counterpart Δ9-THCV, named using the suffix “varin” or “varol”. Such variations are explained by the fact that the geranyl pyrophosphate can be combined with the olivetolic acid and/or the divarinic acid. This is the starting point in the phytocannabinoids' biosynthesis, which results in the formation of the intermediate phytocannabinoids' cannabigerolic acid (CBGA) and/or cannabigevarolic acid (CBGVA) respectively. The intermediate CBGA/CBGVA is subsequently processed by Δ9-THC synthase, which converts CBGA/CBGVA into Δ9-THCA/Δ9-THCVA. Both the proportion between the propyl and pentyl intermediate phytocannabinoids and the presence of Δ9-THC synthase, are genetically determined.
All plants express CBC synthase, which fights for the same intermediate CBGA/CBGVA as CBD synthase and/or Δ9-THC synthase. In ‘normal’ Cannabis plants CBC synthase is active mainly in the early stage, which causes the detection of a higher proportion of this precise phytocannabinoid during the vegetative stage, if compared with the reproductive stage.
The products resulting from the acidic phytocannabinoids degradation, such as CBNA (cannabinolic acid) and CBLA (cannabicyclolic acid) appear as artefacts and are derived from several factors, namely; ultraviolet light, oxidation and isomerisation.
The biosynthetic pathway for phytocannabinoids production is shown in Picture 2.
Basic introduction to the main non-psychoactive phytocannabinoids
The Cannabis plant contains many phytocannabinoids with weak or null psychoactivity, which, from a therapeutic point of view, could be much more promising than Δ9-THC.
CBD is an important non-psychotropic phytocannabinoid that produces a large amount of pharmacological, anti-oxidant and anti-inflammatory effects, among others, transmitted by several mechanisms. It has been clinically proven in cases of anxiety, psychosis and movement disorders, as well as to alleviate neuropathic pain in individuals suffering from multiple sclerosis (it is sometimes combined with Δ9-THC in a 1:1 proportion, as happens in SATIVEX®).
CBDA does not join CB1 and CB2 cannabinoid receptors, although it is an inhibitor of selective COX-2 with anti-inflammatory effects. However, it can join certain vanilloids receptors, but its effects are not fully understood yet. In addition to this, it does act against proliferation.
CBG acts against proliferation and as an antibacterial. It is a ligand from CB2 cannabinoid receptor and an inhibitor of the re-absorption of anandamide. Furthermore, it is a vanilloids ligand.
CBC can cause hypothermia, sedation and hypoactivity in mice. It also acts as an anti-inflammatory, an antimicrobial and a soft analgesic. Moreover, it is a powerful antagonist of vanilloids and a weak inhibitor of the re-absorption of anandamide.