By Viola Brugnatelli

Viola Brugnatelli is a neuroscientist and endocannabinologist, she carries out research and teaching on cannabis at the Dept. Of Neuroscience, University of Padua, Italy, and is the co-founder of
Cannabiscienza, an education company for healthcare professionals on the endocannabinoid system and medical cannabis.
A long standing member of the International Cannabinoid Research Society, and Italian Ambassador for the International Association for Cannabis Medicine, Viola's background is in pharmacology labs, specialising in orphan cannabinoid/terpenoid receptors and their signalling in pain and inflammation.
Currently she collaborates with Fundación Canna, the Journal of Cannabinoid Medicine editorial board, and as a guest author for a series of magazines in the field, including Project CBD. Viola
over the years contributed to several CME courses on cannabis, educating MDs and pharmacists worldwide. She is the VP of a non-for-profit that aims to empower women working with plant medicine.
What fascinates her most about the cannabis plant is what we can learn and understand about the endocannabinoid system and how to modulate it in different ways beyond phytocannabinoids. Lately she has been closely working with anaesthesiologists who operate with hypnotherapy to
evaluate the role of the ECS in the ability of altering our states of consciousness without drugs.
Cannabis phytocomplex
The cannabis plant is a perfect example of a phyto-complex; A plant-based medicine that activates a myriad of different targets.
Many researchers explained the importance of a synergistic activity between minor cannabis components, such as terpenes, phenolic compounds & "minor cannabinoids" in complementing the majors, diminishing their unwanted effects or contributing to improved efficacy. (1, 12)
With its multifaceted physiological interactions, cannabis can be successfully used for a number of different conditions. (2)
This article aims to explore how the poly-pharmacological nature of the Cannabis plant is playing both as the main strength for its re-introduction in medicine, as well as one of the main obstacle for its investigation in scientific research.
What is polypharmacology?
In modern pharmacology the predominant paradigm has been the search for drugs that act as selectively as possible on individual targets: "single molecules" for "single targets". (3)
Our currently-in-use pharmacology is based upon synthesising drugs designed to be very potent & specific on certain targets.
Since these entities would have never existed in nature, it is not always possible to predict their biological interactions once introduced in the human body. (4)
In this mind-frame it's important to avoid "polypharmacology", or the occurrence that a designed molecule may interact with unwanted off-targets, which can cause deleterious side effects & is the number one cause of medicine withdrawal from shelves.
Nevertheless, herbal medicine has been traditionally combining phytocompounds with subtle individual effects, yet with favourable polypharmacology, and enjoyed more clinical success than their highly selective counterparts. (5, 6)
Thanks to the exponential growth of molecular investigation, the "single target" approach has recently shifted into appreciating the complexity of pathways involved with the majority of health conditions, and polypharmacology, (the use of compounds that can engage with multiple targets) is (re)-emerging as the next parameter of drug discovery. (3)
"Re-emerging" because in fact, polypharmacology forms the basis of the majority of traditional medicinal systems, which combine "poly-herbal" (many herbs) preparations, engaging multiple targets in order to achieve better therapeutic effect and reduce the overall toxicity of the treatment. (6, 7)
Whereas developments in analytics methods are supporting a more holistic type of intervention, Cannabis is playing a pivotal point in this emblematic "opening" of modern science to "alternative" phytochemistry. (8)
Single molecule VS whole plant approach
One of the major drawbacks in the implementation of herbal Cannabis in medicine, is the limited availability of double-blind clinical trials run on humans.
To date, most of our clinical knowledge comes from studies carried out on synthetic THC (Marinol) or extracted standardised cannabis in an oromucosal spray (Sativex® & Epidiolex®). (9, 17)
Despite this has greatly advanced our current state of knowledge on safety, titration & symptoms management of specific cannabinoids, one is left to wonder how would our current understanding on herbal cannabis and different chemotypes be, if research weren't exclusively driven by the economic input of companies which aim to patent (& make money) from single compounds of the plant.
It is evident that our current regulatory system does not incentivise the development of multi-component therapies, which is needed for natural herbal products.
It is essential that each compound present is proved to be not only safe, but also efficacious as a single entity. This concept is as deceptive as judging a choir performance by listening to each of the singers individually.
"Besides, with the burden of proof already remarkably high, the development of a product that contains even just two chemicals becomes almost insurmountable for companies that lack substantial R&D budgets". (8, 10)
According to several governments, specific components of the cannabis plant (THC and CBD) have medical value, whilst herbal cannabis is scheduled in international categories that generally designate it as addictive or dangerous and lacking any recognized medical utility. (11, 17)
This type of double-standards seem arguable, especially considering that the most knowledgable researchers of the field report that "the therapeutic impact of the whole plant", with more than 400 compounds found, "is greater than the sum of its single-molecule parts". (12, 19, 20)
What happens when Pharma drives research
Throughout human history medical plants have been of great importance.
Due to the availability of chemical analysis methods in the early 19th century, scientists started to extract and modify active compounds from the plants, resulting in transition from raw herbs to synthetic pharmaceuticals. (13)
Beyond an increase in potency, these newly generated compounds could grant the earning prospect of intellectual property; The first supplied patent in 1790 opened the door to large investments in the pharmaceutical sector, allowing the creators exclusive access to sell their inventions. (5)
A proverbial example of this comes from the active ingredient of willow's bark, salicin, which, similarly to cannabis, has been used for thousands of years by ancient populations (like Sumerians, Egyptians & Greeks) as a painkiller & anti-inflammatory. (12)
Contrary to cannabis though, salicin was the only active compound identified in the plant, and this single molecule has been stabilised into acetylsalicylic acid, and fast patented as Aspirin in 1899 by Friedrich Bayer & Co.
Driven by one, centralised effort, Aspirin has been rapidly launched on the market, and by 1950's it had already become the most sold over-the-counter medicine.
By then, however, there was still no understanding on the mechanism by which this synthetic molecule (Aspirin) interacted with a biologic system (our body), which has been clarified only later in the 1970's thanks to the work of Dr. J.Vane & colleagues. (14)
Our current state of knowledge shows evidence of a medicine with a small therapeutic window, meaning that the therapeutic range of salicylates from Aspirin is 15-30 mg/dl, whilst levels over 90 - 100 usually have serious or life threatening toxicity. (14) In other words, for almost 80 years safety and regulation standards worldwide accepted without a blink that Aspirin be prescription-free and largely available to people, despite ignoring its mechanism of function & the potential interactions & contraindications.
Years later, a recent clinical trial on osteoarthritis using willow bark demonstrated the lack of side effects usually encountered with Aspirin, and a good efficacy of the natural compound possibly due to high salicylic acid content and other plant constituents, confirming how the pharmaceutical choice of manufacturing products might not necessarily represent a superior choice for people's health. (15)
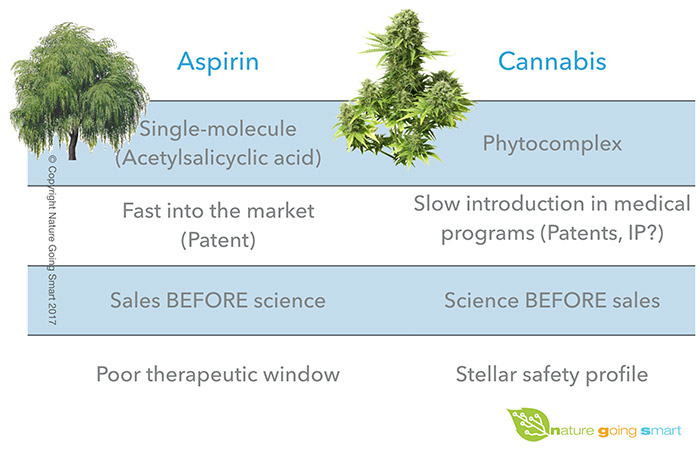
On the other side, despite the stellar safety profile of cannabis, and the amount of scientific literature & case studies on the subject, it is still very difficult to initiate clinical trials with herbal cannabis and regulate its production and sales. (16, 17) This is the challenge that medical cannabis needs to overcome: to put the world "upside-down" and shift from the current paradigm of pharma dictating where the money of research go, and consequently, which drugs doctors are going to prescribe or by which standards our governments should abide.
Patient-inspired research
When healthcare focus shifts towards patients and caregivers, then industry & researchers are finally involved in providing answers for people's well-being.
A patient-inspired approach focuses on the clinical problems and needs of the patient, and leads to therapeutics that are more relevant and straightforward to translate into clinical practice.
There is great need for reliable, consistent data to guide physicians and patients in choosing cannabis chemotypes, administration form and dosage. (17)
Patient survey report that subjective evaluation is more favourable towards herbal cannabis than pharmaceutical products containing cannabinoids, especially for the potential of treating specific symptoms with different varieties. (22)
There are clear advantages on carrying out clinical research on herbal cannabis. The first, and most obvious, is a quiet inexpensive product to obtain which requires little input.
Additionally, there is extensive amount of data showing cannabis safety, and many of the compounds present are already scheduled as dietary supplements. (16, 18)
Moreover, there is great amount of anecdotal evidence and case studies from doctors already using this plant as a medicine to guide research and choose the best parameters to look up.
And lastly, it is probable that embracing the complexity and holistic nature of this plant will support its application in individualized medicine. (8)
Individualized cannabis medicine
By analysing varieties and changes in the plant growth and implementing consistency and standardization based on sound science, clinical research could effectively use the intrinsic polypharmacology of herbal cannabis as an ally in the making of individualized medicines.
By interacting with multiple targets, cannabis can offer the opportunity of treating very different symptoms, different group of people, at different time of the day. Molecules like terpenes can modulate and fine-tune cannabinoids & work synergistically in what has been defined as the "entourage effect". (1, 19, 20)
Thanks to such network approach, it is possible to customise the product to the person, minimise side effects and boost therapeutic benefits.
Despite their complexity, different chemovars can be fully characterised to the same rigorous standard as other pharmaceuticals, designed to conform to a detailed specification dictated by regulatory agencies.
Conclusions
In the past 30 years we have gained a greater understanding of the Endocannabinoid System. However, up until today, most of our clinical research has been carried out with single cannabinoid molecules manufactured either through extraction or synthesis.
This method appears limiting for several factors; New research in network biology confirms that it is more efficient to hit partially multiple targets than to hit completely a single target. (21)
There is clear need for clinical trials with herbal cannabis. To make this possible, the introduction of validated methods for production of quality-controlled varieties is much needed, because the industry is currently saturated with unsafe, contaminated cannabis & products, which may put people's health on the line.
Growing research efforts in the investigation of cannabis entities should be aimed at breeding individualized cannabis medicine.
However, such scenario does not seem realistic in a world which still prevents access to plant therapies.
My hope is that the more educated our collective knowledge on cannabis is, the more the actual chances of creating a ripple effect required for initiating government funding towards patient care rather than Pharma agendas.
References
1) Russo, Ethan B. "Taming THC: potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects". British journal of pharmacology 163.7 2011:1344-1364.
2) Walsh, Zach, et al. "Cannabis for therapeutic purposes: patient characteristics, access, and reasons for use". International Journal of Drug Policy 24.6 2013:511-516.
3) "Network pharmacology: the next paradigm in drug discovery". Hopkins AL Nat Chem Biol. 2008 Nov; 4(11):682-90.
4) Rawlins, M.D. "Variability in response to drugs". Br. Med. J.4, 1974:91–94
5) "What's wrong with drug screening today". Nolan GP Nat Chem Biol. Abril de 2007; 3(4):187-91
6) Jalencas, Xavier, and Jordi Mestres. "On the origins of drug polypharmacology". MedChemComm 4.1, 2013: 80-87.
7) Hasan SS, Ahmed SI, Bukhari NI, Loon WC. "Use of complementary and alternative medicine among patients with chronic diseases at outpatient clinics". Complement Ther Clin Pract. Agosto de 2009;15(3):152-7.
8) Brodie, James S., Vincenzo Di Marzo y Geoffrey W. Guy. "Polypharmacology shakes hands with complex aetiopathology". Trends in pharmacological sciences 36.12 2015: 802-821.
9) Williamson, Elizabeth M. y Fred J. Evans. "Cannabinoids in clinical practice". Drugs 60.6, 2000:1303-1314.
10) Woodcock, J. et al. "Development of novel combination therapies". N. Engl. J. Med. 364, 2011:985–987.
11) Calhoun, S. R., Galloway, G. P. y Smith, D. E. "Abuse potential of dronabinol (Marinol)". J. Psychoactive Drugs 30, 1998:187–196.
12) Russo, Ethan B. y Franjo Grotenhermen, eds. The Handbook of Cannabis Therapeutics: From Bench to Bedside. Routledge, 2014.
13) Tyler, V. E. "Phytomedicines in Western Europe: potential impact on herbal medicine in the United States", in Human Medicinal Agents from Plants, ACS Symposium, No. 534, eds A. D. Kinghorn y M. F. Balandrin (Philadelphia: American Chemical Society), 1993:25–37.
14) Rainsford, Kim D., ed. Aspirin and related drugs. CRC Press, 2016.
15) Schmid, B.et al. "Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: randomized placebo-controlled, double blind clinical trial". Phytother. Res. 2001:15 344–350
16) Sznitman, Sharon R. y Yuval Zolotov. "Cannabis for therapeutic purposes and public health and safety: a systematic and critical review". International Journal of Drug Policy 26.1 2015: 20-29.
17) Russo, Ethan B. "Current therapeutic cannabis controversies and clinical trial design issues". Frontiers in pharmacology 7, 2016.
18) Gertsch, Jürg, et al. "Beta-caryophyllene is a dietary cannabinoid". Proceedings of the National Academy of Sciences 105.26, 2008:9099-9104.
19) McPartland, John M. y Ethan B. Russo. "Cannabis and cannabis extracts: greater than the sum of their parts?" Journal of Cannabis Therapeutics 1.3-4 2001:103-132.
20) Sanchez-Ramos, Juan. "The entourage effect of the phytocannabinoids". Ann Neurol 77 2015:1083.
21) Csermely, P. et al. "The efficiency of multi-target drugs: the network approach might help drug design". Trends Pharmacol. Sci. 26, 2005:178–182
22) Hazekamp, Arno, et al. "The medicinal use of cannabis and cannabinoids—an international cross-sectional survey on administration forms". Journal of Psychoactive Drugs 45.3 2013:199-210.