On February 25 2020, the Center for Disease Control Prevention (CDC), with the U.S. Food and Drug Administration (FDA), state and local health departments, and other clinical and public health partners, released the latest update on the e-cigarette or vaping product use-associated lung injury (EVALI) epidemic. Of 2807 hospitalized cases, 68 were fatal. Fatal lung damage was attributed to vitamin E acetate, which was found both in vaping products and the lung fluids of affected subjects [1].
In November 2019, a case of death associated with the use of e-cigarettes containing Cannabidiol (CBD) was reported in Belgium [2].
OBJECTIVE
The aim of the study is to determine the presence of vitamin E and/or vitamin E acetate as well as other possible harmful substances in commercial samples of CBD-enriched e-liquids marketed in Europe.
METHODOLOGY
Identification tests
Between the end of 2019 and the beginning of 2020, 1 unit (bottle) of 15 different brands of CBD-enriched e-liquids were purchased in different establishments (via the internet, tobacconists, and physical stores).
Each of these bottles was analysed via gas chromatography mass spectrometry (GC-MS) in SCAN mode, which allowed us to obtain information on the total content of the samples. The substances separately detected by the chromatograph were compared with a database (NIST library) that allowed them to be identified with a certain degree of reliability. The identification analysis was carried out with two different chromatographic methods and in different laboratories, which allowed us to detect a wider range of substances present. Likewise, one of the methods employed was carried out using two different injection techniques, direct injection and Headspace.
While the above described tests allow the identification of a large number of substances present in the samples, an additional specific identification test for vitamin E and vitamin E acetate was performed by GC-MS. This test was performed according to the guidelines of the European Pharmacopoeia (04/2005:0439) for the identification of these two substances.
Quantification tests
Once the identification results were obtained, CBD was quantified by liquid chromatography with diode-array detector (HPLC-DAD) and vitamin E acetate by GC-MS. In both methods, samples were analyzed in duplicate.
RESULTS
Identification tests
Most of the samples (14 out of 15) showed the presence of propylene glycol (PG) and glycerin (VG), which are usually the base of e-liquids. One of them contained neither. In the latter, squalane and glycerol tricaprylate, as well as a form of vitamin E, were identified. In specific tests for the identification of vitamin E and vitamin E acetate, this same sample tested positive for the presence of vitamin E acetate.
In addition to these compounds, most of the samples (12 out of 15) contained terpenes that also occur naturally in cannabis plants. The most common were beta-caryophyllene, which was found in 10 of the 15 samples, limonene in 8 samples, and beta-myrcene and farnesene in 7.
Phytocannabinoids other than CBD were also found: d9-THC was identified in all samples and CBDV in 12 of them.
The presence of benzyl alcohol, which can cause coughing, dizziness, and headaches, was identified in one sample by both methods [3].
Quantification tests
With regard to CBD, 14 of the 15 samples showed lower values in the analysis than those declared on the label. Only 4 samples presented values that were less than 10% lower than what was declared (-6%, -3.4%, -2.5% and -8%). On average, CBD was found to be 27% lower than the concentrations stated on the labels, with extremes ranging from +6.67% to -78.33% CBD. Only 1 sample had higher values than declared (6.67%). The coefficient variation (CV%) of the duplicate analysis was very high for some samples (up to 40.03%), indicating a lack of homogeneity of the product.
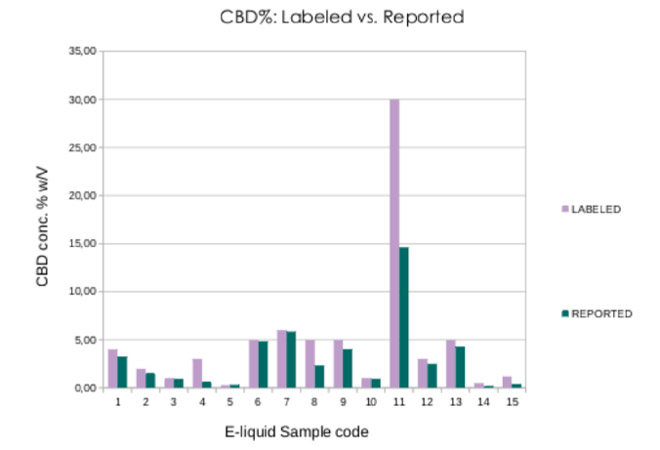
Figure 1. CBD concentration in the samples analysed vs. label claims
The sample that was identified as positive for vitamin E acetate yielded a quantification test result of 244.48 mg of vitamin E acetate for each gram of sample.
CONCLUSIONS
The presence of vitamin E acetate has been confirmed in one of the 15 products examined in this study.
There is no data on how much of this compound can be harmful when inhaled through e-liquids. However, previous literature on the use of these electronic cigarettes and the presence of vitamin E acetate in people with serious lung problems makes its consumption inadvisable.
Similarly, the presence of other compounds such as benzyl alcohol, which can cause short and long term pulmonary problems, highlights the need for greater control over the composition of these products, either by limiting their use or by providing information on the label.
The presence of d-9 THC in all samples means that, depending on the amount consumed, drug tests can yield positive results for this substance.
As for the CBD content, in some samples the deviation between what is declared and what is quantified is very high, as is the lack of homogeneity, showing a lack of controls and good practices when producing the product.
There is also a tendency to add terpenes found in the cannabis plant to the e-liquids, perhaps in an attempt to make the e-liquid taste similar to the plant.
REFERENCES
[1] https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
[2] https://hemptoday.net/vape-rules-europe/
[3] https://pubchem.ncbi.nlm.nih.gov/compound/244
This study has been carried out and financed by the CANNA foundation in collaboration with Phytoplant Research S.L. and the Institut de Recerca i Tecnologia Agroalimentàries (IRTA)