By Tanja Bagar

Dr. Tanja Bagar is a microbiologist with a PhD in Biomedicine. She has gained extensive research experience in biotechnology, molecular biology and cell signaling in laboratories in Slovenia, Germany and the UK. Her focus has mainly been on the endocannabinoid system and active substances from cannabis/hemp. Her work led to the formation of the International Institute for Cannabinoids (ICANNA), where she is the CEO and chairman of the Expert Council. She is also the deputy director and head of R&D in an environmental company. She is active in the academic sphere as well. She lectures on microbiology and is the dean of the master’s program of Ecoremediations at the Faculty Alma Mater Europaea.
By Erhan Yarar

Dr Erhan Yarar is a translational medical doctor with 20 years of experience in paediatric and adult oncology, endocrinology and, lately, in psychiatry. He is a graduate of Hacettepe University/Turkey and has received several certificates from University of Harvard School of Medicine/USA. He has been involved in several medical collaborations in treating patients in diverse parts of the world, such as the USA, Japan, Israel, Germany, Turkey, and Cyprus. He works with alternative and complementary medicine along with conventional medicine. He is an emeritus member of the International Cannabis Research Society and ICANNA. He has experience in applying cannabinoids such as CBD, THC, CBG, and a broad spectrum of terpenes to his patients. He has written many papers, including papers on depression, diabetes, and other medical conditions with respect to the endocannabinoid system and the potential of cannabinoids in medical treatment protocols.
All living beings have daily cyclical patterns, known as 'circadian rhythms'. The name comes from the Latin word 'circa', meaning 'around'. All the beings living on earth are under the influence of day/night cycles and the rotation of the earth around the sun. We all sense these regular external changes and synchronize our physical activities, such as behaviour, food intake, energy metabolism, rest, regenerative movement, and immune function, and thereby extend our chances of survival.
From early life forms onward, we can see ubiquitous intrinsic 'clocks', conserved throughout the evolutionary process, that coordinate the rhythmic physiological and biochemical changes in response to light/dark, as this represents the most dominant and potent stimulus in mammals. Environmental cues, termed 'zeitgebers', determine stage in the cycle, relative to external time, in a process referred to as 'entrainment'. The response of the circadian clock to zeitgebers depends both on the strength of the stimulus and on the circadian phase during which it is applied. Consequently, zeitgebers can advance or delay the circadian clock, thereby ensuring its synchrony with the solar day.

Photo: Institute Icanna
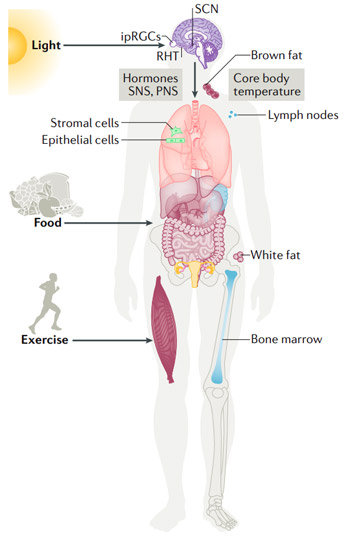
Circadian rhythmicity is created endogenously by genetically encoded molecular clocks or 'clock genes', whose activity and products generate cyclical changes in our physiology with a periodicity of about a day. The circadian clock is genetically controlled, and mutations in clock genes can change rhythmic behaviour in humans, as well as insects, plants, fungi, and bacteria. In essence, the circadian clock is an autonomous, intrinsic timekeeping system, and constitutes an autoregulatory succession of expression, accumulation, and degradation of clock gene products. This in turn creates oscillations in biochemistry, physiology, and behaviours. In animals, the molecular clock controls the expression of genes throughout the body, thereby temporally controlling the activity and function of different cells and organs. Normal circadian physiology is created by a hierarchical network of central and peripheral clocks.
Source: Patke A, Young MW, Axelrod S. Molecular
mechanisms and physiological importance of circadian
rhythms. Nat Rev Mol Cell Biol. 2020 Feb;21(2):67-84.
doi: 10.1038/s41580-019-0179-2.
Epub 2019 Nov 25. PMID: 31768006.
The central circadian pacemaker is found in the suprachiasmatic nuclei (SCN). This is a very small region of the brain located in the hypothalamus, situated directly above the optic chiasm. It receives time-of-day information from light detected by intrinsically photosensitive cells (ipRGCs). Molecular clocks have been shown not only in the SCN, but in virtually all the tissues and cells of the body. These are called 'peripheral clocks', and have been proven to exist in the liver, lung, kidney, heart, skeletal muscles, adipose tissue, and many other tissues. An exception are embryonic stem cells and induced pluripotent stem cells, which seem not to exhibit a functional molecular clock cycle. There is a plethora of evidence that the central clock and the peripheral clocks are essential for the function of organs and the body as a whole.
The information about the internal time-of-day is sent to the rest of the body through hormones, the sympathetic nervous system (SNS), the parasympathetic nervous system (PNS), the core body temperature, and the endocannabinoid system. It has been shown that resonance of internal and environmental time promotes longevity, whereas having an intrinsic cycle that does not match light-dark cycles reduces lifespan.
A misalignment of internal periodicity with the environmental rhythm is deleterious to health and fitness in all species, and research shows that a mismatched clock is worse than none at all.
The interplay between the endocannabinoid system and the circadian rhythm
The organs and tissues throughout the body that have central and peripheral circadian clocks also have a functional endocannabinoid system; they express cannabinoid receptors and/or produce endocannabinoids. The expression of cannabinoid receptor 1 (CB1) has already been proven in the hypothalamus, including the SCN, and the presence of other components of the endocannabinoid system have been shown in other systems, organs, and tissues in the body. Endocannabinoids are lipophilic molecules that act as retrograde signal molecules and are produced by the postsynaptic cell. After they enter the synaptic cleft, they activate cannabinoid receptors on the presynaptic cell to regulate neuronal excitability. Recent evidence strongly suggests that astrocytes play an important role in this retrograde signalling as a means of fine-tuning the responses to cannabinoids. In the SCN, the central circadian pacemaker, astrocytes not only tune the retrograde responses but also have an active role in integrating time-of-day information to the SCN network.
Endogenous cannabinoid signalling is recognized as a ubiquitous signalling system that coordinates intercellular communication in multicellular organisms. It has been shown that the ECS and its manipulation by exogenous cannabinoids affects sleep/wake cycles, temperature regulation, food consumption and fat storage, central nervous system (CNS) regulation of autonomic and endocrine functions, reward-driven behaviour, gastrointestinal function, mood, and sensory perception. All of these processes have a cyclical circadian rhythm. The ECS has a modulatory role and serves as a means of maintaining the homeostatic intracellular environment; in a way, it functions as a guardian of our inner balance.
ECS is intertwined with the circadian rhythm in several respects, some pointing to 'downstream' and other to 'upstream' regulation. The amount of endocannabinoids, their degradative and synthetic enzymes, and their receptors all show tissue-specific diurnal changes, indicating that ECS is 'downstream' of circadian regulators. On the other hand, exogenous and endogenous cannabinoids affect many important physiological processes that exhibit a circadian rhythm: sleep-wakefulness, body temperature, HPA endocrine secretions, food intake, learning and memory, and locomotor activity. These findings indicate that ECS is 'upstream' of circadian processes. Recent research evidence suggests that the ECS serves as a link between the circadian regulators (intrinsic clocks) of the suprachiasmatic nucleus and the following physiological responses.
Interestingly, the ECS itself shows rhythmic behaviour. Specific cyclic patterns have been reported in three major components of the ECS, namely the endocannabinoid tissue content, CB1 receptor expression, and in the enzymes controlling the synthesis and degradation of endocannabinoids, further underlining a close correlation with the circadian cycle. Diurnal rhythmic oscillations have been well-documented for anandamide, where healthy humans have a threefold higher plasma concentration on waking than immediately before sleep, a dynamic that becomes dysregulated with sleep deprivation. Anandamide is generally found in low concentrations in the hippocampus in sleep and higher in the active phase. Interestingly, the hippocampal content of 2-AG was higher during the inactive phase and lower in the active, and a similar dynamic has also been found for the CB1 receptor. When cannabinoid receptors are activated, the body's circadian responses change; CB1 activation blocks light-induced phase shifts of circadian behaviour and increases neuronal firing within the SCN by decreasing presynaptic GABA release.
Implications in sleep
There is an interesting connection of the two systems in the pineal gland, a brain region that synthesizes and releases melatonin, a serotonin-derived hormone which modulates sleep patterns in both circadian and seasonal cycles. This gland receives multi-synaptic signals from the suprachiasmatic nucleus – the central circadian clock – and expresses all the ECS components. The CB1 receptors are present in both pinealocytes and on the terminals of sympathetic afferents to the gland, and enzymes for synthesis and degradation of the endocannabinoids of the ECS are expressed in the pineal gland. Cannabinoids are known to influence the production of melatonin, since tetrahydrocannabinol (THC) and other plant-derived cannabinoids reduce melatonin synthesis in the pineal via a non-CB1 receptor-dependent mechanism.
There is ample evidence that ECS affects the organization of sleep. It is involved in the control of the sleep/wake cycle. Available data indicate that ECS maintains and/or promotes the sleep state. Anandamide causes a decrease in wakefulness and an increase in slow wave sleep (SWS) and rapid eye movement sleep (REMS). Research has also shown that acute administration of THC causes a decrease in REMS and increases slow wave sleep. On the contrary, chronic administration has been found to decrease SWS with inconsistent effects on REMS. Further evidence in support of a role for the ECS in the regulation of sleep comes from studies demonstrating that CB1 receptor density is significantly increased during the rebound phase following sleep deprivation. This indicates that increased ECS at the level of the receptor may be involved in the homeostatic recovery after sleep deprivation.
In line with this recent research, data suggests that cannabinoids could be very useful for sleep disturbances. Tetrahydrocannabinol (THC) can decrease sleep latency but could impair sleep quality long-term if used chronically. Cannabidiol (CBD) may have therapeutic potential for the treatment of insomnia, especially for REM sleep behaviour disorder and excessive daytime sleepiness. Synthetic cannabinoids, such as nabilone and dronabinol, have been shown to be beneficial in obstructive sleep apnea due to their modulatory effects on serotonin-mediated apneas. Nabilone has also been shown to reduce nightmares associated with PTSD and to improve sleep among patients with chronic pain.
Conclusions
Many physiological processes are regulated by circadian rhythms, and more and more data is showing that dysregulation of circadian rhythms, or a mismatch between circadian rhythmicity as occurs in modern human society, contributes to human diseases. ECS seems to be a pivotal link between the circadian clocks and the following responses, showing bidirectional relationship between ECS signalling and circadian processes. Cannabinoids, both plant-derived and synthetic, show immense potential to regulate the imbalances in the circadian rhythmicity and sleep. With their basic balancing function, cannabinoids modulate the activity of the SCN and the central circadian pacemaker, as well as other circadian clocks throughout the body, contributing to general health and wellbeing.
There is an especially interesting interplay between the ECS, the circadian rhythmicity, and sleep. Since insomnia and other sleep disturbances are becoming a highly prevalent condition that is associated with increased health risks, the potential of cannabinoids to aid sleep is of great importance. Insomnia is the most common sleep disorder, reported in ∼30% of adults, and chronic insomnia in about 10%. Prevalence of obstructive sleep apnea is also high, with estimates of 9-21% in women and 24-31% in men. Evidence suggests that cannabinoids used at the right time, and in the appropriate concentration and composition, can be of great benefit to inducing sleep and correcting the architecture of sleep, possibly due to their modulatory effects on the circadian clocks, thereby contributing significantly to the health, well-being, and productivity of the individual and, considering how prevalent they are, also to society.
References
Vaughn LK, Denning G, Stuhr KL, de Wit H, Hill MN, Hillard CJ. Endocannabinoid signalling: has it got rhythm? Br J Pharmacol. 2010 Jun;160(3):530-43. doi: 10.1111/j.1476-5381.2010.00790.x. PMID: 20590563; PMCID: PMC2931554.
Acuna-Goycolea C, Obrietan K, van den Pol AN. Cannabinoids excite circadian clock neurons. J Neurosci. 2010 Jul 28;30(30):10061-6. doi: 10.1523/JNEUROSCI.5838-09.2010. PMID: 20668190; PMCID: PMC2927117.
Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. 2020 Feb;21(2):67-84. doi: 10.1038/s41580-019-0179-2. Epub 2019 Nov 25. PMID: 31768006.
Hablitz LM, Gunesch AN, Cravetchi O, Moldavan M, Allen CN. Cannabinoid Signaling Recruits Astrocytes to Modulate Presynaptic Function in the Suprachiasmatic Nucleus. eNeuro. 2020 Feb 13;7(1):ENEURO.0081-19.2020. doi: 10.1523/ENEURO.0081-19.2020. PMID: 31964686; PMCID: PMC7029187.
Babson KA, Sottile J, Morabito D. Cannabis, Cannabinoids, and Sleep: a Review of the Literature. Curr Psychiatry Rep. 2017 Apr;19(4):23. doi: 10.1007/s11920-017-0775-9. PMID: 28349316.