By Lisette Wijnkoop / Arno Hazekamp

Lisette Wijnkoop was educated as a pharmacist and have been working a lot in the field of natural medicines like homeopathy. In 2006 she started with cannabis as medicine in a specialised cannabis pharmacy in Groningen in The Netherlands. This project ended after one year and she started to work at Bedrocan, both as a quality controller of the processing of cannabis and as an adviser for patients, doctors and others in the medical field. At this moment Lisette is adviser for the medicinal cannabis division of Information Center Cannabis, an independant organisation. With Arno Hazekamp and others they are starting a foundation to organise courses for medical doctors and give information to other groups interested in medicinal use of cannabis.
Arno Hazekamp

Dr. Arno Hazekamp (1976) studied molecular biology (Bachelor degree) and biopharmaceutical sciences (Masters degree) at Leiden University, the Netherlands. From 2001-2007 he focused his PhD research on the medicinal aspects of the cannabis plant, and was actively involved in setting up the Dutch medicinal cannabis program, working closely with Bedrocan and the Office of Medicinal Cannabis. In the period 2007-2010 Arno further specialized as a medical cannabis expert, working closely with government agencies, universities and small pharmaceutical companies. From 2009-2013, Arno was a board member of the International Association for Cannabinoid Medicines (IACM). He is an active traveller and lecturer, and is considered a professionally trained medicinal cannabis advocate. In 2011, Arno became the Head of Research and Education (R&D) of Bedrocan BV. Currently, his main focus is on setting up the Dutch Expertise Center for Cannabis (DECC) where he works as an independent consultant. Arno has published over 30 scientific papers on various aspects of medicinal cannabis.
Over recent years, cannabis has been rapidly changing from a recreational drug to a medicine. In other words, an unregulated product used for fun or adventure is turning into a medicine for seriously ill patients in need of a reliable product. This transition has important consequences for the quality standards that we should expect from cannabis products.
For example, patients usually do not want to become high. Instead, they want to function well enough to get out of the house, drive a car, or visit with family. For them, getting high is mostly an unwanted side effect. On the other hand, patients want to take enough cannabis to feel the desired therapeutic effect. That means the quantity of THC present in a cannabis product is important to know, in order to give the patient an idea about the right dosage. Additionally, patients may have a weakened immune system. They must be protected against harmful additives such as pesticides, harmful micro-organisms and fungal toxins produced by certain moulds.
To know what a product contains as active ingredients while also being sure it does not contain any harmful additives is important for all products being used as a medicine. Cannabis is no exception to this. This goal can be achieved by a combination of proper standardization and reliable quality control of cannabis products.
Product standardisation and quality control
In the Netherlands, as part of the official Medicinal Cannabis Program supervised by the Office of Medicinal Cannabis, there is a certified and legal cultivator for medicinal cannabis. To guarantee a safe quality and a constant level of active ingredients, a lot of measures are taken during the cultivation process. It starts with the way of multiplying the plants: by making cuttings, also known as cloning, every plant is genetically the same. When working with seeds, every plant can have another composition of ingredients thus leading to different effects. Differences between batches of identical genetic strains may also be caused by cultivation conditions, such as type and amount of light, temperature, nutrients or humidity. And finally, the way of processing, drying and storage of the plants can further influence the composition of the active ingredients in cannabis. This means all steps from cultivation to delivery to the patient need to be sufficiently controlled.
In The Netherlands, medicinal cannabis is tested by an independent lab for appearance (bud shape and size), cannabinoid content, terpene profile and water content. And the absence of hair, insects, bacteria, and moulds are tested, as well as heavy metals, pesticides and fungal toxins. All steps together guarantee that Dutch medicinal cannabis is standardised and quality-controlled.
These are the conditions that apply for the cannabis legally cultivated under GMP conditions and tested in an official lab. This cannabis is standardised plant material and can be sold as a pharmaceutical grade cannabis. But is this all necessary in case of making products, such as cannabis oils, or the preparation of CBD-products, that are often brought on the market as a dietary supplement?
It is important to realize that no matter what legal category a cannabis product is, it is always important that a product contains all the ingredients that are on the label and does not contain any harmful additives. So the question is: What exactly should be analysed and labelled? The most important is the amount of the most potent active ingredients, the cannabinoids. On the label must be clearly mentioned what the content is of the major cannabinoids, which should at least include THC, THCA, CBD, and CBDA, and preferably also CBN, CBG and delta-8-THC. All these compounds may have a significant but different pharmacological effects.
What is unwanted in a cannabis product?
The most important requirement for health products is that they are safe(1) to use by the consumer. This can be realised by following proper (hygienic) procedures and excluding the addition of toxic substances by using safe and non-toxic materials only. To ensure product safety, in-process controls can be applied during the manufacturing and the final product should be examined in a good, and preferably independent lab.
Pesticides can be present in plant material because growers may use it to control or eradicate pests such as insects or moulds. As cannabis cultivation is often not controlled (i.e. not inspected by independent organizations or agencies), a lot of pesticides can and are used. In a recent study in the Netherlands(2) on 25 samples obtained from coffeeshops, 23 contained detectable amounts of pesticides. Unfortunately, it is not well known precisely how harmful pesticides are when they are smoked. However, it has been shown they can be inhaled and will enter the lungs directly while smoking contaminated cannabis(3). For food products, certain maximum concentrations of pesticides are allowed, but it should be noted that this is based on oral consumption. The maximum allowed concentration of pesticides in oral dosage forms of cannabinoid products can perhaps be compared to these regulations.
Bacteria and moulds can develop easily under the warm and humid conditions used for cannabis growing, particularly during indoor hydroponics cultivation. The presence of E.coli, an intestinal bacteria, is a signal of bad hygienic circumstances. Certain moulds, such as the species Aspergillus flavus, produce highly toxic products (called aflatoxins) that are tolerated to be present in only extremely low levels in food products(4).
Heavy metals such as cadmium, lead or mercury can unintentionally be present in soil, water or fertilizers, and end up in the plant. EU-guidelines and pharmaceutical regulations provide maximum levels and describe tests to analyse these heavy metals in food and medicines.
Sometimes adulterants can be added to cannabis buds, to give them more weight (for example small lead particles or ground up glass) or make them look better (for example talcum powder). Visual and microscopic inspection of cannabis, especially of unknown origin, can help to detect this(5).
Methods used to analyse cannabinoids
The many different cannabinoids can be difficult to distinguish from each other during laboratory testing. After all, there are so many cannabinoids and they have a lot of overlapping properties. Apart from good quality and very costly equipment, such as HPLC or GC, experienced staff is necessary to use such complex devices in a proper way. Even with proper equipment, different results can be obtained on the same product, when it is sent to different laboratories(6). The reason is that the analytical machine is only part of the lab. Training, maintenance, calibration, and the use of validated testing protocols are some other essential factors. Every lab is responsible for making sure all these requirements are met.
The first step during the testing of cannabis or a cannabis product is to make a solution where all the ingredients to be tested are present. A good solvent has to be chosen and the procedure to follow has to be 'validated'. That means it is proven that by following the procedure, the same and correct result will be obtained, every time again. It is a bit similar to calibrating a scale when you want to make sure it indicates the right weight. Without calibration, the scale may always give the same result during weighing, but the result is always wrong.
To separate all the different cannabinoids from each other, often advanced techniques have to be used. Most common are gas chromatography (GC) and high-performance liquid chromatography (HPLC). GC works mainly on the difference in evaporating temperature of different cannabinoids (and terpenes too), while HPLC depend more on the solubility of cannabinoids in different solvents. After successful separation, the subsequent step will be the detection of which and how much of a substance comes out of the 'separation tube'.
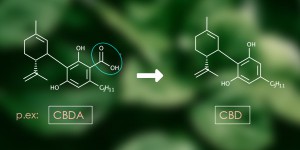
When GC is used for separation of the cannabinoids, detection techniques include e.g. mass spectrometry (MS) and flame ionization detection (FID). When using GC, the solution to be analysed is heated to a high temperature of up to 250°C. At such temperatures, the cannabinoids will convert from their acid form, into their neutral from (for example: THC-acid (THCA) becomes THC, CBD-acid (CBDA) becomes CBD etc.). The consequence is that only the total content of acidic plus neutral cannabinoids will be measured in GC.
In contrast, HPLC is a separation method used at room temperature, so that the cannabinoid acids (THCA, CBDA, CBGA, etc) can be detected separately from their neutral forms (THC, CBD, CBG, etc). HPLC is often combined with a UV- or photodiode array (PDA) detector. Validated methods using HPLC for the analyses of cannabinoids are described in several official guidelines including the American United States Pharmacopoeia (USP), but these are not intended to be used for whole cannabis plant materials. For the Dutch medicinal cannabis program a validated method was developed for quality control using ultra-performance liquid chromatography (UPLC). This method is a more recent version of the HPLC that can analyse cannabinoids in a shorter time. Unfortunately, this method is also more expensive. GC is a good method for analysing cannabis buds. It measures both acid and free forms of the cannabinoids together. This total gives the potential THC and CBD content when used for inhalation with a vaporizer or smoking. For final products, like oils or tinctures, the HPLC or UPLC method is better. The free and acid forms of the cannabinoids are measured separately so the THC, THCA, CBD and CBDA content can all be separately specified on the label.
Conclusion
A lot of products made of cannabis (high THC) and of hemp (low THC) are now sold on the market. These new products demand new quality standards. Because cannabis extracts have a complex composition which make them difficult to analyse, good laboratories are needed, well equipped and with properly trained technicians. Standards for the quality control of whole cannabis plant material and extract are not yet well defined. This leads to risks of unsafe products getting in the hands of patients. Most important is the responsibility of the producer to label his product correctly and clear so that the costumer understands what he is buying and using. Laboratories should learn to be open to communicate about their methods and their degree of validation and open to improve their skills.
This sounds like a lot of work, but it will ultimately be good for the trust in your products and the future of cannabis medicine.
Literature:
(1) Lisette Wijnkoop, Cannabis as herbal medicine-Rules and regulations, www.fundacion-canna.es/en/cannabis-herbal-medicine-rules-and-regulations
(2) RIVM (Rijksinstituut voor Volksgezondheid and Milieu (Dutch Institute for Health and Environment), Cannabis contaminanten, 2015
(3) Nicolas Sullivan, Sytze Elzinga and Jeffrey C. Raber, Determination of Pesticide Residues in Cannabis Smoke, Journal of Toxicology, 2013
(4) http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32006R1881 Reglamento (CE) Nº 1881/2006 de 19 de diciembre de 2006 por el que se fija el contenido máximo de determinados contaminantes en los productos alimenticios
(5) Arno Hazekamp, An introduction to Medicinal cannabis, 2016
(6) Dale Gieringer and Arno Hazekamp, How accurate is potency testing? O'Shaughnessy's, Otoño de 2011
Lisette Wijnkoop and Arno Hazekamp. Both writers are board members of the Dutch scientific information centre for cannabis (WIC)