By Raquel Peyraube

Dr. Raquel Peyraube is a doctor in medicine and a specialist in the problematic use of drugs. She has trained in psychiatry, toxicology and psychoanalytical psychotherapy and in subjects such as childhood, adolescence and social exclusion. She has 28 years' experience in the field. Throughout her career, she has made contributions in training, prevention, treatment and damage reduction, including innovating theoretical and methodological developments with emphasis on ethical issues. As a former clinical director of ICEERS, she is now an ad hoc consultant of the Uruguayan National Drugs Secretariat giving advice on reform of the public drugs policy and of the Institute of Cannabis Regulation and Control. She is a clinical researcher and a member of research teams for monitoring the law in Uruguay. She sits on several international scientific committees and is an active member of the IACM (International Association for Cannabinoid Medicines). She currently works on the development of clinical trials, medical education on medicinal cannabis, and dissemination of information and advice for reform of drugs policies in various countries.
Idiopathic pulmonary fibrosis (IPF), also known as diffuse interstitial pulmonary fibrosis, is an incurable disease with no known cause (i.e. an idiopathic condition). As its name suggests, it is characterised by diffuse fibrotic scarring of the lung tissue. This process leads to the healthy lung tissue being replaced with fibrotic tissue; as result, the lung loses its elasticity, becoming rigid. Progress of the fibrotic process gradually impairs respiratory function to the point where it is severely compromised, as the tissue is not fit to perform the gas exchange (delivery of oxygen and elimination of carbon dioxide) involved in the respiratory function.
IPF is a very uncommon disease. It is estimated that it affects around 5 million people throughout the world and it is slightly more prevalent among men than women. Onset usually occurs between 55 and 66 years of age, although it can start earlier in some cases. Some forms of the disease are familial.
The cause or causes of the disease are still unknown. Nor is it known why some people are affected and others are not, even when exposed to the same factors that have been postulated as being possible triggers of its genesis. Nonetheless, some information is available and certain hypotheses have been put forward regarding its etiopathogenesis. Multiple causes have been postulated, ranging from genetic components, environmental pollutants, infection, exposure to certain medicines and tobacco smoke.
Symptoms include progressive respiratory difficulty as the disease advances. Blood oxygenation levels gradually fall, initially in common activities and then even with the most minimal movements. This evolution is generally rapid, taking between some months and some years, but in some cases, it is much slower.
Other symptoms include a (generally dry) cough, fatigue and fever. When oxygenation levels fall, cyanosis appears (i.e. a bluish discolouration of lips and nails). As in other pulmonary conditions, clubbing of the fingers may occur.
Pulmonary fibrosis with the loss of the fine lung vasculature eventually affects the heart as the result of an increase in pressure in the pulmonary arteries which the heart must overcome to pump blood to the lung for oxygenation.
Treatment is oriented towards relieving the symptoms and trying to delay evolution of the disease. Basically, two drugs are used which have been approved for treating IPF: pirfenidone and nintedanib. As the disease advances, patients will require an oxygen supply at home and portable devices to provide oxygen wherever they go. Respiratory rehabilitation and physiotherapy can help relieve the symptoms for a time. A lung transplant may be considered in some cases.
A study was presented at the ICRS conference in Montreal in June 2017, which I consider to be particularly important given the present state of our knowledge and the therapeutic possibilities for treating IPF. At present, we have little to offer such patients, who have a very delicate prognosis.
I found the study especially interesting as I have had two consultations regarding this disease. Patients quite often consult their physicians about the possibility of using additional cannabis-based treatments when their prognosis is unpromising and when treatment has proved either ineffective or has many adverse side effects.
My clinical reasoning had already led me to consider using cannabidiol, given its powerful anti-inflammatory action, despite the fact that assessments of anti-inflammatory treatment have not shown it to reduce the fibrotic response in this pathology (this is probably because the mechanism whereby the fibrosis is activated does not appear to be a response to a triggering inflammatory process).
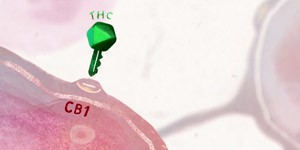
One group of American researchers have proposed the CB1 receptor as a therapeutic target for treating IPF in association with another therapeutic target to try to improve antifibrotic effectiveness (Cinar R et al1). Endocannabinoids acting by way of the CB1 receptor are known to promote fibrosis at a hepatic, cardiac and renal level and in radiation-induced pulmonary fibrosis. Given that the role of these compounds in IPF is unclear, the authors tried to identify its role in the complex and multifactorial pathogenesis of this disease, and hypothesised that it might be possible to improve the therapeutic effectiveness by addressing several of the mechanisms involved.
Inducible nitrous oxide synthase (iNOS) activity is increased in IPF and this increase correlates with the progress of the disease. Inhibitors of this enzyme have shown to have an antifibrotic effect in animal models (mice).
In their research, the authors assessed endocannabinoid activity and the activity of the CB1 receptor and iNOS, both in humans and in the animal model equivalent. They also assessed the therapeutic potential in mice of an orally administered synthetic molecule that inhibits both the CB1 receptor and iNOS. The study was conducted on human and animal lung samples. The researchers found that anandamide increased both in patients with IPF and in animals in which pulmonary fibrosis was induced, and that this increase was accompanied by a worsening of the pulmonary function (measured using tests of pulmonary function). In the animal lungs, this increase was correlated with a worsening of the pulmonary function. The findings confirmed a reduction in the expression of FAAH (Fatty Acid Amine Hydrolase), an enzyme that degrades anandamide, which evidently contributes even more to raising these levels. They also found a significant and mutually independent elevation in levels of CB1 and iNOS in patients with severe IPF. Likewise, CB1 activation in macrophages (active cells in inflammatory processes) was associated with a proinflammatory and profibrotic state, and an increased expression of interferon regulatory factor 5.
Simultaneous blocking of the CB1 receptor at a pulmonary level and of iNOS with the experimental molecule administered dramatically improved animal survival rates. The authors conclude that simultaneous blocking of the two increases the therapeutic antifibrotic effectiveness.
However, as already stated, treatments with anti-inflammatories in IPF patients have not given the results that would be expected of a fibrosis secondary to an inflammatory process. In other words, if the fibrosis were the result of an inflammatory process, as commonly occurs, inhibiting the inflammatory response with anti-inflammatories should improve the fibrosis.
Obviously, there is much that much remains to be clarified about the mechanism involved in IPF, but the results of this study open a window of hope to patients, in that it appears that simultaneous blocking of iNOS and CB1 might improve the effectiveness of antifibrotic treatment. We might therefore be on the threshold of a treatment for these patients, which although not curing them, would at least slow the evolution of the disease significantly as compared to the treatments currently available.
At the same time, CBD has been shown to modulate the levels of anandamide to a greater or lesser degree in different pathological processes. Despite the lack of scientific evidence of its potential beneficial effect in IPF, it is reasonable to wonder whether because of its mechanisms of action, CBD might also have this simultaneous twin action at a pulmonary level and particularly in IPF: downregulation of CB1 receptors because of its action as a CB1 antagonist, combined with its action in reducing the expression of proinflammatory enzymes, including precisely iNOS through NFκB antagonism (a core potentiating factor of the kappa light chains of activated B cells) through activation of the PPAR-γ receptors.
In view of safety levels in the use of CBD (when properly medicated and indicated, it has a low rate of adverse effect, generally ranging from slight to moderate). Depending on the absence or availability of effective treatments, I consider that doses of cannabidiol of between 100 and 300 mg per day might be added to patients' current treatments, depending on their tolerance and their financial possibilities.
Given the absence of legal regulation for such products in most countries, their medicinal use is also unsubsidised. Unfortunately, this means that economic factors have a bearing on patients' ability to access products of pharmaceutical quality in sufficient doses to treat their conditions.
Bibliography
1. Cinar R, Gochuico BR, Iyer M, Yokoyama T, Park JK, Coffey NJ, Jourdan T, Gahl WA and Kunos G. Laboratorio de Estudios Fisiológicos de NIAAA y Medical Genetic Branch de NHGRI
Fernández-Ruiz J, Saagredo O, Pazos, MR, García C, Pertwee R, Mechoulam R, Marínez-Orgado J. Cannabidiol for neurodegenerative disorders:important new clinical applications for this phytocannabinoid? Br J Clin Pharmacology. 2013 Feb; 75(2):323-333 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3579248/ (14 de agosto de 2017)
Esposito G, Sucderi C, Valenza M, Togna GI, Latina V, De Filippis D, Cipriano M, Carratú MR, Iovone T, Sreardo L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3230631/ (14 de agosto de 2017)
Esposito G, De Filippis D, Maiuri MC, De Stefano D, Carnuccio R, Iuvone T. Cannabidiol inhibits inducible nitric oxide sythase protein expression and nitric oxide production in beta-amiloid situmalted PC12 neurons throug p38 MAP kinase and NF-kappaB involvement. http://www.sciencedirect.com/science/article/pii/S0304394006000681?via%3Dihub (14 de agosto de 2017)
Rajesh M, Mukhopadhyay R, Bátkai S, Haskó G, Liaudet L, Drel VR, Obrosova IG, Pacher P. Cannabidiol attenuates high glucose-induced endotelial cell inflammatory response and barrier disruption. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2228254/ (14 de agosto de 2017)