By Carlos Goicoechea

Professor of Pharmacology at URJC. Ph.D. in Pharmacology from UCM. Coordinator of the "Research Excellence in Pain" Group at URJC- Santander (2014-2017), coordinator of the Pharmakom High Performance Experimental Pharmacology Research Group at URJC, and member of the Working Group on the Basic Science of Pain and Analgesia of the Spanish Pain Society (2018-2022). Director of the official Master's course "The Study and Treatment of Pain" (2007-2010). Vice-president of the Spanish Pain Society.
Author of 85 articles (of which 55 in international journals indexed by JCR), and researcher in 31 projects and 27 contracts (of which 15 as principal investigator). 122 participations in national congresses (of which 78 as invited speaker) and 113 in international congresses (of which 22 as invited speaker), 75 lectures given, 18 Doctoral Theses supervised (of which 3 "Extraordinary Doctorate Award"). 34 book chapters. 13 research awards (of which 2 international).
Recognized as a chronic disease in the latest International Catalog of Diseases by the WHO (https://icd.who.int), pain can be defined as primary or secondary, which means it can be the consequence of a demonstrated physiological alteration (secondary) or have an undetermined (primary) origin. However, no matter whether it's primary or secondary, there is growing certainty that pain, in particular chronic pain, is a disease, and as such should be treated individually, while of course continuing to treat the pathology that caused it.
The approach to chronic pain is not easy. And it can't be. It involves a disease in which not only physiological but also psychological factors converge. Economic, social and cultural factors also play a role. All this means that if all these factors aren't addressed simultaneously, it's very likely (almost certain) that the final result will leave much to be desired. But it will not be easy. We are not prepared for this, we don't have the habit, nor the strategy, nor the necessary tools to approach pain from all these diverse perspectives.
To understand the complexity of the approach and treatment of pain, we have to briefly review the nociceptive system and the pathophysiological changes that occur as a consequence of a nociceptive stimulus over a longer period of time. Distinguishing between pain and nociception is very important when it comes to understanding our ability to modulate pain from a pharmacological standpoint.
The nociceptive system and pain
The presence of a nociceptive stimulus tells us that something is not working properly. That something can be external (such as a puncture, a burn) or internal (for example a fracture, an infection). In both cases, the body has a highly specialized system that detects, transmits and modulates (to some extent) this information, alerting to a potentially harmful stimulus; this is the nociceptive system. It's composed of neurons that send the message to the spinal cord and, from there, to the brain. In the brain, the location and intensity of the stimulus are identified, it acquires its emotional components and, when it reaches the prefrontal cortex, the information is interpreted and becomes pain (Goicoechea and Martín, 2006).
There are endogenous pain control systems: neurons that are capable of modulating the transmission of the nociceptive signal to decrease the intensity of the message that reaches the brain. These systems are located in the spinal cord. Some are part of local spinal circuits, but, in other cases, they are pathways that are generated in the brain, neurons that descend to the spinal cord to stop nociceptive communication (D'Mello and Dickenson, 2008). These descending pathways cannot be activated voluntarily, but they can be stimulated for example by moderate physical exercise, positive expectations and psychological training.
The nociceptive system reports any situation that can be harmful and dangerous to the integrity of the individual, which is why it provokes an unpleasant response, so that an animal or person can escape, flee the situation and protect itself; or avoid certain behavior in the future. It also creates a memory to avoid someone from putting themselves in that same situation again; pain and memory always go hand in hand, for better and for worse (Fairhurst et al., 2012; Ji et al., 2003).
Pain is like an alarm. Pain relief systems are never (or almost never) 100% effective, because the maintenance of pain is essential to our survival; it reminds us there is something we must avoid or fight. Furthermore, if the alarm signal does not switch off, everything will focus on amplifying the activation of the nociceptive system, exaggerating the information and amplifying its transmission, to further turn up the alarm (Basbaum et al. 2009).
That is when a series of changes occur that transform pain into a disease in itself, in a process that evolves independently of the cause where it originated, with as its ultimate goal to increasing the amount of information that reaches the brain, where it's interpreted in the prefrontal cortex.
When pain becomes chronic, the nociceptive system is completely modified: the pathways (neurons) responsible for transmitting nociceptive stimuli increase their effectiveness, and the endogenous systems responsible for controlling this information become less efficient (Ji et al., 2018). And the emotions generated in the limbic system are exaggerated, amplifying the entire system (McCarberg and Peppin, 2019). Chronic pain patients don't experience their pain, or their surroundings, in the same way, which of course changes their life significantly.
Therefore, the pharmacological approach to pain must take into account the entire complexity of chronic pain, and adapt to its characteristics (type of pain, duration, location, etc.). For this we can use anti-inflammatory analgesic drugs, paracetamol, opioids, antidepressants (here not used to treat depression, but to enhance the downward modulating systems) and neuromodulators (Schug & Goddard, 2014).
The choice of pharmacological therapy has to be personalized and adapted to the type of pain and the characteristics of the drugs, but also to the patient of course. The beneficial effects of pain treatment may be the result of its effect on the nociceptive pathways (peripheral effects), or on the perception that the patient has of these stimuli, how he copes with the pain experience (central effects).
The endocannabinoid system and pain
The endocannabinoid system is an inhibitory neurotransmitter system. This means it's composed of substances that interact with receptors capable of decreasing the activity of neurons. These are substances (such as anandamide, diacyl-glycerol) that are generated 'on demand' and decrease neuronal activity (Gómez-Ruiz et al., 2007).
The endocannabinoid system is present throughout the entire nociceptive system: there are CB1 cannabinoid receptors on nociceptors and on spinal and brain neurons. That is why, ever since its discovery in the middle of the last century, attempts have been made to stimulate this system pharmacologically to reduce the transmission (nociceptors) and interpretation (brain) of pain (Martín Fontelles and Goicoechea García, 2008).
In addition to being present in nociceptive neurons, we find cannabinoid receptors (CB2) in the cells of the immune system (Bie et al., 2018). These cells that are activated when they detect harm or danger and that provoke an inflammatory response, generally, create a greater nociceptive response (because they stimulate receptors located in the nociceptors, always with the 'goal' of associating harm and pain, to generate a harm-avoidance memory). And there is also the neuroimmune system, the glial cells (microglia and astrocytes) where cannabinoid CB2 receptors are also present and that are responsible, in the Central Nervous System, for neuroinflammation that contributes to the chronification of pain (Wang, 2019 ).
Right from the beginning, the use of cannabinoid agonists in lab animals has demonstrated that the activation of the endocannabinoid system produced analgesic effects (antinociceptive, to be more precise) (Lynch, 2005). Animals treated with these drugs showed a high pain response threshold, which means they took longer to respond to pain. This was verified in different models of animal pain (acute and chronic, nociceptive and neuropathic) (Pascual et al., 2005; Burgos et al., 2010). From the start, it was also found that stimulating the endocannabinoid system caused psychoactive effects (effects on the brain), identified as those induced by the recreational use of cannabis (Fride et al., 2006).
It's precisely these effects on the brain that have posed a problem when developing cannabinoid drugs for pain treatment. The treatment of chronic pain involves continuous drug use to try to lead a 'normal' life as much as possible, so the psychoactive effects can be a very relevant problem when using these drugs in chronic patients.
However, there is an interesting possibility to pharmacologically modulate the endocannabinoid system without causing psychoactive effects, by affecting the CB2 receptors located in the immune system and the glial cells of the spinal cord. These pro-inflammatory cells that also contribute to chronic pain, can be effectively inhibited with CB2 receptor agonists (Beltramo, 2009).
Although as of today, no synthetic cannabinoid agonist has proven to be a highly effective analgesic, at least at the clinical level, there is much interest in developing selective cannabinoid agonists that can deliver a good analgesic result with few side effects, because they selectively target either the CB2 receptors or the CB1 receptors, without having access to the Central Nervous System (Cumella et al., 2012; Mulpuri et al., 2018).
Cannabis and pain
One of the first medicinal uses of hemp was in pain treatment. And it still is. One of the first recommended used for medicinal cannabis is pain treatment, although there is still some controversy, at least in some areas, about its effectiveness as an analgesic treatment.
The use of the plant in pain treatment has become widespread in recent years. There are numerous forms of consumption (smoking, inhaling, ingesting, topical application in the form of a cream,) and a variety of plants are used with varying concentrations of active ingredients. (Grant et al., 2012). This makes it extremely difficult to be certain of how cannabis works on pain, to differentiate whether it actually acts on the perception of pain itself, or in the way someone perceives and interprets any external sensation.
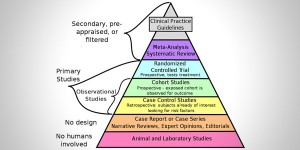
The method that allows us to verify if a medicine, a treatment, or a technique, is effective in treating a pathology, is a clinical trial. Statistically supported clinical evidence is not the same as clinical experience, based on daily use and treatment. Clinical experience should form the basis for the working hypotheses that are developed in the clinical trial. This is a required step to confirm that the observation can be applied to a much larger group of patients, and to enable the development of treatments with broad therapeutic use and few (or acceptable) side effects.
Although the situation is changing rapidly, there are not many (at least not enough) well-designed clinical trials that allow us to analyze the real effect of cannabis in managing different types of pain. As already mentioned, differences in the formulations, the type of patient, the duration of treatment and other factors and variables (for example, it's not the same to assess only the subjective intensity of pain, but quality of life as well), make it very difficult to draw universal conclusions (Whiting et al, 2015; Schrot et al., 2016).
We are not conducting a review of every study published so far; those considered most useful or relevant are included in the bibliography. However, we can put forward a series of conclusions.
There is insufficient evidence to use cannabis in acute pain situations, although low-quality studies have indicated that its analgesic effects are superior to a placebo (Gazendam et al., 2020). A higher number of studies have been published on chronic pain and their conclusions indicate that cannabis can be used in those situations in which satisfactory results have not been achieved with other treatments for which more evidence is available (First et al., 2020, Fisher et al., 2019). It's more effective than a placebo, but the side effects, although usually not serious, we can't neglect their frequency and characteristics. Frequent side effects include: sedation, confusion and disorientation, among others. One of the pains that causes the most suffering in patients is neuropathic pain or nerve pain, which is difficult to treat due to the complexity of its pathophysiology. In this type of pain, cannabis produces a moderate analgesic effect, with side effects that can be tolerated in most cases, depending on the severity of the pathology (Mücke et al, 2018). In cancer pain, as in other pains, the efficacy is moderate and still disputed (Meng et al., 2020), although its use may be beneficial because it can also reduce some of the side effects of cancer treatment with chemotherapy, such as nausea and vomiting (Chung et al., 2020).
Countries where medicinal cannabis is already being used for the treatment of pain propose its use in carefully selected patients, always under strict medical supervision and with proper knowledge of the origin of the product used (Ko et al., 2016).
In conclusion, there clearly is a 'void' that can be filled by the use of cannabis and cannabinoids in the treatment of chronic pain, in situations where pain is not reduced with other drugs or the side effects of these drugs are unacceptable for the patient. Efficacy depends highly on the patient, the pathology and the product used. We cannot draw general conclusions at this time. Side effects, although generally not serious, are not uncommon and affect the cognitive sphere. These require more well-designed studies that not only compare the use with placebo but also with other treatments to better define a more precise scope for its use (Moore et al., 2020).
| TYPE OF PAIN | Results | CONCLUSIONS |
| Acute pain | There is insufficient evidence that its analgesic effects are superior to a placebo. The side effects can be significant. | Use is not recommended for this type of pain. |
| Neuropathic pain (nerve pain) | Moderate analgesic effect with tolerable side effects in most cases. | It can be used when other treatments aren't effective (third/fourth line). |
| Oncological pain (cancer pain) | Moderate analgesic effect, in addition to relevant antiemetic (reduces nausea and vomiting) effect for this type of pain. | For patients who are resistant to other treatments. |
| Chronic pain (non-oncological, non-neuropathic) | Little evidence and often equal results to placebo. | Use is not recommended. |
| Nociception, nociceptive system | This system is responsible for transmitting a potentially harmful stimulus, from the periphery to the higher centers of the Central Nervous System. |
| Nociceptors | Neurons (nerve cells) responsible for detecting harmful stimuli and transmitting these to the spinal cord. |
| Receptor | A protein or a series of proteins, generally located on the surface of the cell, which serves to activate the nociceptor in response to various potentially harmful stimuli. |
| Spinal cord | The structure that produces the integration (and modulation) of all the information coming from the periphery |
| Somatosensory cortex | Nervous system located in the brain that is responsible for identifying the location and intensity of the nociceptive stimulus originating in the periphery. |
| Limbic system | Nervous system located in the brain responsible for generating emotions associated with peripheral stimuli (can be nociceptive or not). |
| Prefrontal cortex | Nervous system where all the information from the different brain structures is integrated and where pain is generated. |
| Immune system | Defense system formed by different cells (such as lymphocytes, mast cells or mastocytes, macrophages) that uses an inflammatory response to respond an attack or harm (both external and internal). |
| Neuroimmune system | Cells known as glia or glial cells (such as astrocytes, microglia) that, stimulated by the neurons of the central nervous system, can release pro-inflammatory substances that contribute to the chronification of pain. |
| Neurotransmitter | Substance generated by a neuron that can bind to receptors located in other neurons to modulate their activity. These can either be excitatory (if they increase neuron activity) or inhibitory (if they decrease activity). |
| Agonist | Substance (natural or synthetic) that is able to bind to a receptor and stimulate it, causing a response in the cell. |
| Nociceptive pain | Pain, which can be muscular, bone or visceral pain, caused by the existence of an identifiable harmful peripheral stimulus. |
| Neuropathic pain | Pain caused by the existence of a lesion in the somatosensory nervous system, which is responsible for transmitting and regulating sensations (pain, but also touch, temperature and others). |
Bibliography:
Basbaum AI, Bautista DM, Scherrer G y Julius D. Cellular and molecular mechanisms of pain. Cell. 2009; 139(2):267-84.
Beltramo M. Cannabinoid type 2 receptor as a target for chronic - pain. Mini Rev Med Chem. 2009 Jan;9(1):11-25. doi: 10.2174/138955709787001785. PMID: 19149657.
Bie B, Wu J, Foss JF y Naguib M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol. 2018; 31(4): 407-414.
Burgos E, Pascual D, Martín MI, Goicoechea C. Antinociceptive effect of the cannabinoid agonist, WIN 55,212-2, in the orofacial and temporomandibular formalin tests. Eur J Pain. 2010; 14(1):40-8.
Chung M, Kim HK, Abdi S. Update on cannabis and cannabinoids for cancer pain. Curr Opin Anaesthesiol. 2020; 33(6):825-831. doi: 10.1097/ACO.0000000000000934. PMID: 33110020.
Cumella J, Hernández-Folgado L, Girón R, Sánchez E, Morales P, Hurst DP, Gómez-Cañas M, Gómez-Ruiz M, Pinto DC, Goya P, Reggio PH, Martin MI, Fernández-Ruiz J, Silva AM, Jagerovic N. Chromenopyrazoles: non-psychoactive and selective CB₁ cannabinoid agonists with peripheral antinociceptive properties. ChemMedChem. 2012; 7(3):452-63.
D'Mello R y Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101(1):8-16.
Fairhurst M, Fairhurst K, Berna C y Tracey I. An fMRI study exploring the overlap and differences between neural representations of physical and recalled pain. PLoS One. 2012;7(10):e48711.
Fisher E, Eccleston C, Degenhardt L, Finn DP, Finnerup NB, Gilron I, Haroutounian S, Krane E, Rice ASC, Rowbotham M, Wallace M, Moore RA. Cannabinoids, cannabis, and cannabis-based medicine for pain management: a protocol for an overview of systematic reviews and a systematic review of randomised controlled trials. Pain Rep. 2019; 4(3):e741.
Fride E, Perchuk A, Hall FS, Uhl GR y Onaivi ES. Behavioral methods in cannabinoid research. Methods Mol Med. 2006; 123:269-90.
Gazendam A, Nucci N, Gouveia K, Abdel Khalik H, Rubinger L, Johal H. Cannabinoids in the Management of Acute Pain: A Systematic Review and Meta-analysis. Cannabis Cannabinoid Res. 2020 Dec 15;5(4):290-297.
Goicoechea C y Martín MI. Mecanismos periféricos y centrales del dolor. Reumatol Clin. 2006;2 Supl 1:S5-9
Gómez-Ruiz M, Hernández M, de Miguel R y Ramos JA. An overview on the biochemistry of the cannabinoid system. Mol Neurobiol. 2007; 36(1):3-14.
Grant I, Atkinson JH, Gouaux B, Wilsey B. Medical marijuana: clearing away the smoke. Open Neurol J. 2012; 6:18-25.
Ji RR, Kohno T, Moore KA y Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003; 26(12):696-705.
Ko GD, Bober SL, Mindra S, Moreau JM. Medical cannabis - the Canadian perspective. J Pain Res. 2016; 9:735-744.
Lynch ME. Preclinical science regarding cannabinoids as analgesics: an overview. Pain Res Manag. 2005;10 Suppl A:7A-14A.
Martín Fontelles MI, Goicoechea García C. Role of cannabinoids in the management of neuropathic pain. CNS Drugs. 2008;22(8):645-53.
McCarberg B y Peppin J. Pain Pathways and Nervous System Plasticity: Learning and Memory in Pain. Pain Med. 2019; 20(12):2421-2437.
Meng H, Dai T, Hanlon JG, Downar J, Alibhai SMH, Clarke H. Cannabis and cannabinoids in cancer pain management. Curr Opin Support Palliat Care. 2020; 14(2):87-93. doi: 10.1097/SPC.0000000000000493. PMID: 32332209.
Moore RA, Fisher E, Finn DP, Finnerup NB, Gilron I, Haroutounian S, Krane E, Rice ASC, Rowbotham M, Wallace M, Eccleston C. Cannabinoids, cannabis, and cannabis-based medicines for pain management: an overview of systematic reviews. Pain. 2020 May 28. doi: 10.1097/j.pain.0000000000001941. Epub ahead of print. PMID: 32804833.
Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018; 3(3):CD012182.
Mulpuri Y, Marty VN, Munier JJ, Mackie K, Schmidt BL, Seltzman HH, Spigelman I. Synthetic peripherally-restricted cannabinoid suppresses chemotherapy-induced peripheral neuropathy pain symptoms by CB1 receptor activation. Neuropharmacology. 2018; 139:85-97.
Pascual D, Goicoechea C, Suardíaz M, Martín MI. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain. 2005; 118(1-2):23-34.
Schrot RJ, Hubbard JR. Cannabinoids: Medical implications. Ann Med. 2016;48(3):128-41. doi: 10.3109/07853890.2016.1145794. Epub 2016 Feb 25. PMID: 26912385.
Schug SA y Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-75.
Wang J. Glial endocannabinoid system in pain modulation. Int J Neurosci. 2019; 129(1):94-100.
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. Erratum in: JAMA. 2016 Apr 12;315(14):1522. PMID: 26103030.