By Tanja Bagar

Dr. Tanja Bagar is a microbiologist with a PhD in Biomedicine. She has gained extensive research experience in biotechnology, molecular biology and cell signaling in laboratories in Slovenia, Germany and the UK. Her focus has mainly been on the endocannabinoid system and active substances from cannabis/hemp. Her work led to the formation of the International Institute for Cannabinoids (ICANNA), where she is the CEO and chairman of the Expert Council. She is also the deputy director and head of R&D in an environmental company. She is active in the academic sphere as well. She lectures on microbiology and is the dean of the master’s program of Ecoremediations at the Faculty Alma Mater Europaea.
The ECS and metabolism
As we are learning more and more about the endocannabinoid system, it is becoming increasingly evident that the narrow definition of this system no longer applies or accurately describes the complexity of this regulatory system. We know that there are many endocannabinoids produced in our bodies, that we have more than two cannabinoid receptors, and that phytocannabinoids have many molecular targets in our cells and tissues. As a result, some researchers and authors now use an extended definition of the endocannabinoid system, such the "enlarged or expanded endocannabinoid system" or simply "endocannabinoidome". This term represents the ensemble of endocannabinoids, endocannabinoid-like mediators, and their many receptors and metabolic enzymes.
The endocannabinoidome is involved in controlling many processes in our bodies and, overall, the role of this system is very complex. It affects the majority of the physiological systems, and the cannabinoid receptors are expressed (in different densities) on all studied cell types. So, describing what exactly it does is not an easy task, as it regulates the biochemistry of the vast majority of 37 trillion cells in our body. Research has shown that the endocannabinoid system functions as an SOS mechanism that is activated whenever our bodies are out of balance for whatever reason. For example, it is activated when we suffer from a physical injury, when we encounter pathologic microbes, and when we feel emotional pain or are under stress. It seems that the ECS serves as a general protective mechanism, starting at the cellular level, and proceeding to the tissues, organs, body, and our general well-being.
Unsurprisingly, the endocannabinoidome is involved in all aspects of our metabolism, including appetite, energy balance, metabolism, thermogenesis, inflammation and nociception, as well as regulation of stress, reward, and emotions. As the prevalence of metabolic disorders, including obesity, is constantly increasing, scientists are looking for new therapeutic strategies, including expanded ECS. The components of the endocannabinoidome are emerging as potent therapeutic targets due to their role in the regulation of food consumption and energy balance, as well as lipid and glucose metabolism. Studies have shown that the ECS is upregulated during obesity and associated metabolic disorders. The levels of endocannabinoids are elevated in obese individuals in comparison to lean. Increased concentration of endocannabinoids has been shown in the central nervous system, adipose tissue, pancreas, skeletal muscle, kidney, liver, and blood of obese rodents and humans. The cause is not very clear, but could be due to enhanced synthesis of endocannabinoids or their reduced degradation as well as overexpression of the cannabinoid receptors. Various studies have shown upregulated levels of 2-AG in different organs and serum during obesity and hyperglycemia, which was correlated with body fat content, visceral fat mass, and fasting plasma triacylglyceride and insulin concentrations. Similarly, several studies revealed substantial association between AEA level and body mass index (BMI) value.
The activation of the CB1 receptor is involved in controlling lipid and glucose metabolism. Even though CB1 is principally expressed in the nervous system and its expression levels are low in peripheral cells, it increases in obesity. The blockade of CB1 reduces food intake and body weight. Consequently, Rimonabant, an antagonist at CB1, was developed and also prescribed as an anti-obesity drug, but it had severe side effects including depression, anxiety, nausea, dizziness, and suicidality, probably due to the blockade of central CB1. There is considerable evidence about the negative impact that CB1 has on thermogenesis. It was observed that CB1-lacking mice have less fat and are more protected against obesity than the corresponding wild-type mice. CB1 receptor is known for its appetite-stimulating effects due to the binding of THC to this receptor, producing so-called "munchies". The effects of cannabis on increasing appetite have been known for a long time, with historical sources indicating that people as early as 300 BCE knew that cannabis stimulated appetite, especially for sweet and savoury food. CB2 also plays a role in feeding; in particular, its agonists can reduce food intake.
Adipose tissue
Metabolic disorders, including insulin resistance and obesity, are emerging as leading health concerns all over the world. These conditions are characterized by excessive or abnormal fat accumulation, the so-called adipose tissue, and are major risk factors for various chronic diseases, such as cardiovascular diseases, diabetes, and generally poorer quality of life.
Adipose tissue is commonly distinguished as white and brown adipose tissue according to their appearance. Brown adipose tissue (BAT) is characterized by small lipid droplets and high density of mitochondria, which leads to the brown appearance. White adipose tissue (WAT) cells usually show just one lipid droplet. The morphologic differences also reflect different functions. BAT is involved in thermogenesis and caloric expenditure during resting and exercise. WAT is involved in fat storage and endocrine secretion of hormones. In response to various stimuli, WAT can start to show the characteristics of the brown adipose cells that are called beige or brite adipocytes.
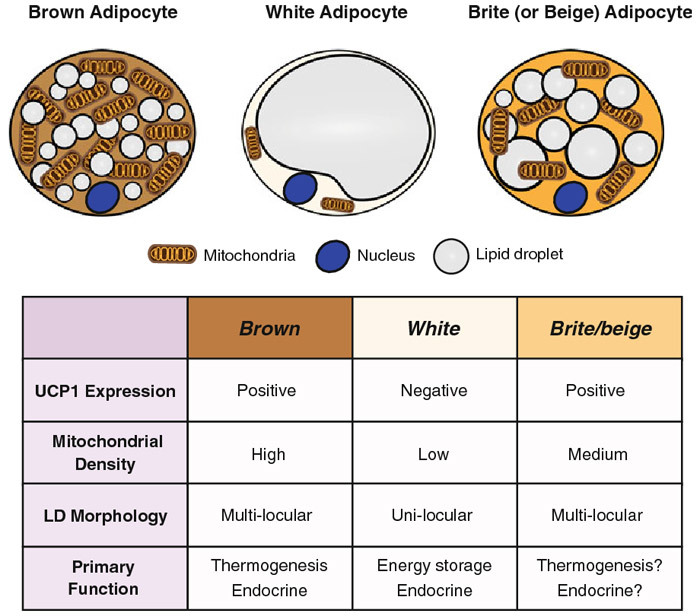
Photo source: Jung et al., 2019
More and more research shows that the induction or recruitment of beige or brite adipocytes, together with the activation of BAT, could be protective against obesity. BAT activity has the potential to significantly influence body weight, glucose, and lipid metabolism. It is involved in dissipating energy as heat, due to a special protein called UCP1 on the inner mitochondria membrane. Brown adipocytes seem to be responsible for large heat production, significantly higher than in other organs, thereby enhancing body energy expenditure. Several activators have been associated with WAT browning, like cold exposure, exercise, thyroid hormones, catecholamines, capsaicin etc. The conversion of WAT in beige adipose tissue is a potential new therapeutic target for obesity.
Adipose tissues express both cannabinoid receptors, and their activation significantly influences the metabolic activity of adipose cells. Blockade of the CB1 receptor was shown to induce the differentiation of white adipocytes towards a thermogenic brown cell phenotype, which is highly beneficial when we want to reduce adipose tissue. The effects of the CB2 receptor on obesity are poorly characterized, but we do know it is strongly involved in energy homeostasis. It was shown that the activation of CB2 induces heat generation with consequent energy expenditure in adipose tissue and that chronic stimulation of CB2 attenuates body weight gain. Therefore, CB2 seems like a promising target in obesity, as it should facilitate the anti-obesity effects without exerting central psychotropic activity.
Tetrahydrocannabinol (THC)
THC is a partial agonist of both CB1 and CB2 receptors and is the psychoactive constituent of cannabis, due to its binding to the CB1 receptor in the brain. Additionally, THC activates CB1 receptors, located in the limbic (enhancing motivational properties of food) and hypothalamic (increasing appetite) regions of the brain, causing acute appetite stimulation or-so called orexigenic effects. On the other hand, it was shown to have potential anti-obesity effects. In an in vivo model, researchers demonstrated that chronic administration of THC to diet-induced obesity (DIO) mice for 3 and 4 weeks prevented weight gain due to reduced energy intake. THC was also reported to exhibit a beneficial impact on the regulation of insulin sensitivity in the insulin-resistant adipocytes. The mechanisms of these dual effects are not fully elucidated, but since THC does not produce maximal stimulation of CB1 receptors, one explanation could be that the observed anti-obesity effects could result from blocking full agonist anandamide from binding to the CB1 receptor, especially since the endocannabinoid tone is high in obesity. However, the potential positive influence of THC in the prevention and treatment of obesity requires further investigation.
Tetrahydrocannabivarin (THCV)
THCV binds to both cannabinoid CB1 and CB2 receptors. Its action seems to be dose-dependent, as THCV is a neutral antagonist at low doses and agonist at high doses of CB1 and CB2 receptors. As it has CB1 antagonistic action, it seemed to be a promising candidate for metabolic disorders and obesity. Research has confirmed some of this, as THCV was shown to have a potential therapeutic effect in the treatment of diabetes associated with obesity. THCV increased insulin sensitivity and improved glucose tolerance in diet-induced obese mice as well as genetically obese mice. THCV also restored intracellular insulin-signalling pathway and decreased lipid accumulation in the liver and adipocytes. A pilot study in patients with type 2 diabetes showed that THCV reduced fasting plasma glucose with parallel improvement in β-cell function in the pancreas. Therefore, this phytocannabinoid should be strongly considered when metabolic disorders need to be addressed.
Cannabidiol (CBD)
CBD has a wide range of well-researched pharmacological effects through many different mechanisms. It is a negative allosteric modulator of CB1 receptor, blocking the binding of strong agonists such as anandamide, thereby producing anti-obesity effects. Since, in obesity, ECS is overactive, there is an overflow of anandamide and 2-AG, causing overactivation of the CB1 receptor. At the CB2 receptor, it can act as an agonist or inverse agonist depending on the dose and research model. CBD also has affinity for various other receptors, including GPR55, α1-adrenoreceptors, 5-HT1A (serotonin), TRPV channels, and PPARγ. CBD displays many therapeutic properties, including anti-inflammatory, anti-oxidant, anti-tumour, anti-convulsant, and neuroprotective effects. Due to these, CBD emerges as a potential therapeutic agent, which can be used in the treatment of diabetes and its complications –obesity, ischemia, neurodegenerative diseases – as well as relieving pain and depression. Since obesity is associated with chronic low-grade inflammation, CBD can play a crucial role in downregulating this inflammation response and aiding healthier body mass. In this respect, CBD has been studied extensively and was shown to enhance the level of glutathione (GSH), adenosine triphosphate (ATP), and nicotinamide adenine dinucleotide (NAD), and to increase intracellular lipolysis and mitochondrial activity (36). CBD has many beneficial effects on the liver, which is a key organ in all metabolic processes and energy balance, since it attenuates liver steatosis (decreased triacylglyceride and fat droplet accumulation), inflammatory response, nitrative stress, reduced lipid peroxidation, expression of reactive oxygen species, and neutrophil infiltration in the liver. The effects of CBD on food intake, food preferences, and weight gain have been ambiguous and showed an array of confounding results. One study demonstrated that CBD caused a decrease in body weight gain in rats, while other studies have shown no significant impact on food intake and body weight in mice and rats. One study also showed that CBD in rats fed a high fat diet resulted in an increase in body weight despite significantly reduced food intake. Regardless of the contradictory results of CBD on feeding, CBD shows manifold beneficial effects on a diverse array of changes and pathologies involved in obesity. Since it binds to many receptors, it produces effects on many organs crucial in metabolism, like the liver, adipose tissue, the pancreas, and the heart. Interestingly, it also shifted eligible changes in gut hormones, such as GIP—glucose dependent insulinotropic peptide and adipokines concentrations.
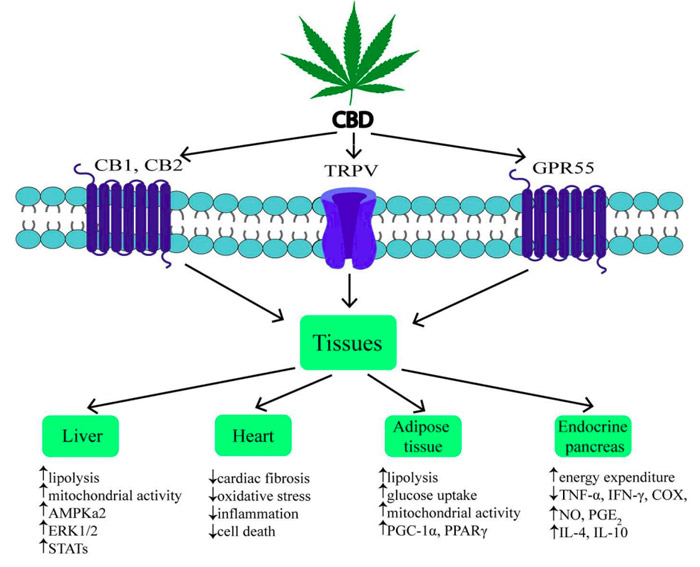
Photo source Bielawiec et al., 2020
Conclusion
Obesity-related co-morbidity is increasing. It comes at a high cost for society and it has a negative impact on a patient's lifestyle. Recent findings in the field of white adipose tissue browning (WAT to BAT transformation) have pointed out the potential role of this phenomenon in obesity treatment. BAT activity has been associated with the improvement of metabolic profile in adults. The activation of CB2 in enhancing browning and transforming chemical energy into thermic energy could be an important piece of the puzzle.
Targeting of CB1 is also important, but we must bear in mind the experiences with Rimonabant, a CB1 receptor antagonist that appeared to be a promising anti-obesity drug during clinical trials, but also exhibited immense psychiatric side effects and had to be taken off the market. The discontinuation of clinical trials related to FAAH inhibitors because of serious adverse events has also shown that meddling with this vital, fundamental protective system needs to be done very judiciously. Here, once again, phytocannabinoids and also other ligands, the cannabinoid receptors from the plant world, show immense potential. The focus is on finding CB1 receptor antagonists and CB2 receptor agonists in the plant world. They show a high safety profile with negligible side effects and have multiple targets within the endocannabidiome that support healthy metabolism, energy expenditure, biochemical homeostasis, and emotional well-being.
Literature
Jung SM, Sanchez-Gurmaches J, Guertin DA. Brown Adipose Tissue Development and Metabolism. Handb Exp Pharmacol. 2019;251:3-36. doi: 10.1007/164_2018_168. PMID: 30203328; PMCID: PMC7330484.
Bielawiec P, Harasim-Symbor E, Chabowski A. Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Front Endocrinol (Lausanne). 2020 Mar 4;11:114. doi: 10.3389/fendo.2020.00114. PMID: 32194509; PMCID: PMC7064444.
Rossi F, Punzo F, Umano GR, Argenziano M, Miraglia Del Giudice E. Role of Cannabinoids in Obesity. Int J Mol Sci. 2018 Sep 10;19(9):2690. doi: 10.3390/ijms19092690. PMID: 30201891; PMCID: PMC6163475.
Borowska M, Czarnywojtek A, Sawicka-Gutaj N, Woliński K, Płazińska MT, Mikołajczak P, Ruchała M. The effects of cannabinoids on the endocrine system. Endokrynol Pol. 2018;69(6):705-719. doi: 10.5603/EP.a2018.0072. PMID: 30618031.
Kaur R, Ambwani SR, Singh S. Endocannabinoid System: A Multi-Facet Therapeutic Target. Curr Clin Pharmacol. 2016;11(2):110-7. doi: 10.2174/1574884711666160418105339. PMID: 27086601.
Behl T, Chadha S, Sachdeva M, Sehgal A, Kumar A, Dhruv, Venkatachalam T, Hafeez A, Aleya L, Arora S, Batiha GE, Nijhawan P, Bungau S. Understanding the possible role of endocannabinoid system in obesity. Prostaglandins Other Lipid Mediat. 2021 Feb;152:106520. doi: 10.1016/j.prostaglandins.2020.106520. Epub 2020 Nov 26. PMID: 33249225.
Abioye A, Ayodele O, Marinkovic A, Patidar R, Akinwekomi A, Sanyaolu A. Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes. J Cannabis Res. 2020 Jan 31;2(1):6. doi: 10.1186/s42238-020-0016-7. PMID: 33526143; PMCID: PMC7819335.


