By Guillermo Moreno-Sanz

Dr. Moreno-Sanz has authored more than 30 scientific articles and 3 patents describing the role of the endocannabinoid system in pain perception. Graduated in Biochemistry and Organic Chemistry from the University of Zaragoza, he obtained his PhD in Neuroscience from the Complutense University of Madrid, in Spain. He gained extensive international experience with long-term fellowships in the Netherlands, Italy, and the United States, developing most of his academic career at the University of California, Irvine, where he discovered a new class of cannabinoid analgesic with high clinical potential. In 2017, he acted as a consultant to the National Academies of Sciences of the United States in the preparation of the report "The health effects of cannabis and cannabinoids" and later founded Abagune Research to offer scientific advice and R&D solutions to the international cannabis industry. In 2020 he assumes the scientific and medical direction of Khiron Life Sciences in Europe.
The plant kingdom has always been an inexhaustible source of medicinal resources for humanity. Traditional medicine treatises are replete with herbal preparations for various disorders and conditions. However, with the development of modern medicine, a new discipline has been making its way since the mid-19th century: medicinal chemistry. Medicinal chemistry is dedicated to the chemical modification of lead molecules to improve their therapeutic profile, either by reinforcing their medicinal properties (potency, efficacy, etc.) or by reducing their adverse effects (toxicity, selectivity, etc.). We can therefore affirm that there is a relationship between the structure of a molecule and its pharmacological activity (namely, structure-activity relationship or SAR).
One of the first examples of this new discipline was the discovery of aspirin. The white willow (Salix alba) has been used by different civilizations for medicinal purposes, appearing in the texts of classical doctors such as Hippocrates, Dioscorides or Galen as a natural relief for pain and fever. The active principle from willow bark was isolated in 1828 by Johann Buchner, professor of pharmacology at the University of Munich, in the form of yellow and bitter crystals that he called salicin. Ten years later, the Italian chemist Raffaele Piria obtained salicylic acid through the chemical transformation of salicin. In 1853, French chemist Charles Frédéric Gerhardt obtained acetylsalicylic acid by trying to improve the bitter taste and gastric irritation caused by salicylic acid. However, it would not be until 1897 when Felix Hoffmann, a pharmacist at Bayer laboratories, achieved the synthesis of high purity acetylsalicylic acid. Two years later, the German pharmaceutical company began its commercialization under the trade name "Aspirin", becoming the first drug in the group of non-steroidal anti-inflammatory drugs (NSAIDs) which also include other commonly used drugs such as ibuprofen, naproxen, or indomethacin.1
This example helps us to illustrate the differences between the three types of molecules that I wanted to talk about in this article. Preparations of Salix alba´s bark and their active principle, salicin, are natural substances, since their structure has not undergone any modification with respect to the way they are found in nature. Salicylic acid and acetylsalicylic acid obtained in the mid-1800s are semisynthetic compounds, as they are analogues that start from a natural molecule whose structure has been modified to improve its activity. The acetylsalicylic acid obtained by Felix Hoffman is a synthetic molecule, since its preparation does not start from a natural substance, but from other simpler chemical compounds. Like aspirin, the rest of NSAIDs are also synthetic molecules and, although their structures are completely different, they share the same pharmacological activity, that is, they all inhibit the enzyme cyclooxygenase (COX). Similarly, we can draw a distinction between natural, synthetic, and semisynthetic cannabinoids.
Natural cannabinoids
By now, most of us know the usual suspects: the main actors (Δ9-THC and CBD), their acidic precursors (THCA and CBDA), the supporting actors that are going strong (CBG, CBN, Δ8-THC) and the extras with promising potential (CBGA, CBC, THCV, CBDV). When one begins to speak of natural cannabinoids a diffuse number, between 104 and 150, of compounds of this family that have been identified in some variety of cannabis is commonly given. We will later talk about some of these minor cannabinoids that have been characterized recently, but the truth is that, probably due to their low relative abundance, these compounds do not seem to contribute significantly to the effect of cannabis and its derivatives.
It should be noted that natural cannabinoids are currently the molecules approved for clinical use capable of modulating the endocannabinoid system. Their natural origin makes them almost impossible to patent or protect intellectually except for two exceptions: i) when they are combined in a particular ratio that presents a differential effect, such as the 1: 1 ratio of THC and CBD in nabiximols, or ii) when they are formulated in a defined and immutable presentation, as is the case with the drug Epidiolex, a syrup that contains purified CBD, and has been proved effective for some forms of pediatric refractory epilepsy.
There are also regulatory reasons why phytocannabinoids have been produced synthetically. In their synthetic version, both THC and CBD are not subjected to the same restrictive legislation that makes their natural analogues difficult to use because they came from a prohibited or highly controlled plant. The recent decriminalization of natural CBD for certain purposes will benefit its production from low-THC cannabis compared to synthetic alternatives based on limonene obtained from citrus peel or biosynthetic approaches using genetically modified microorganisms.
Synthetic cannabinoids
Despite the synthetic versions of THC and CBD and for the sake of simplicity, we can say that synthetic cannabinoids are those molecules that are not structurally related to natural cannabinoids and have been produced in medical chemistry campaigns aimed to obtaining compounds capable of selectively activating cannabinoid receptors, both CB1 and CB2 alike. The names of these molecules usually begin with an acronym of the chemist who designed them as Alexandros Makriyannis (AM) or John W. Huffman (JWH) who, in his group at Clemson University, produced more than 450 synthetic and semisynthetic cannabinoids. The acronym may also belong to the institution in which the studies that produced these compounds were carried out such as the Hebrew University of Jerusalem (HU), home to Professor Raphael Mechoulam, creator of some famous representatives of these families of molecules.
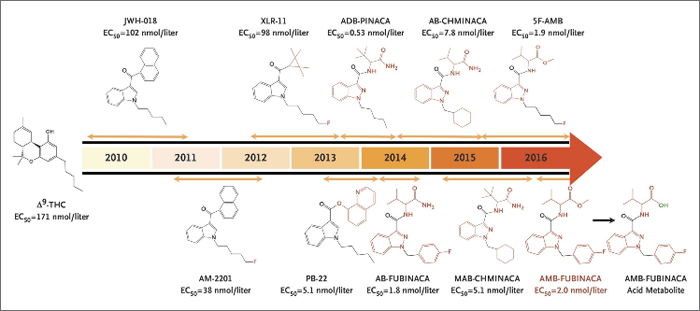
Figure 1. Synthetic cannabinoids: Turning youth into zombies since 2010. Figure from Adams et al., 2017.
Figure 1 represents a temporal evolution of how these compounds have been adopted for exploitation as drugs of abuse, being produced by organizations halfway between Walter White and the Russian mafia, and marketed in the form of herbal preparations onto which these synthetic cannabinoids are sprayed. Every time the health authorities identify and ban one, a new one takes its place in products known as "Spice" or "K2". They are generally very potent agonists of the CB1 receptor, which also have the characteristic of being total agonists (THC is a partial agonist) that can therefore exacerbate the adverse effects associated with the central activation of CB1, giving rise to grotesque episodes such as the "zombie attack" that occurred in New York in the summer of 2016.2
Semi-synthetic cannabinoids
The major difference between synthetic and semi-synthetic cannabinoids is that the latter maintain the chemical structure of THC, on which small chemical modifications are produced in order to improve or refine its pharmacological profile. The advantage of semi-synthetics is that they start from a molecule whose activity is already known and, therefore, it is possible to rationalize what the effect of the chemical modification or modifications will be as the SAR study progresses. Figure 2 shows some of the chemical groups (also called "residues") that are most relevant to the activity of THC and how modifying them can affect its pharmacological profile.3 We will focus on three of these positions: C2, C3 and C9.
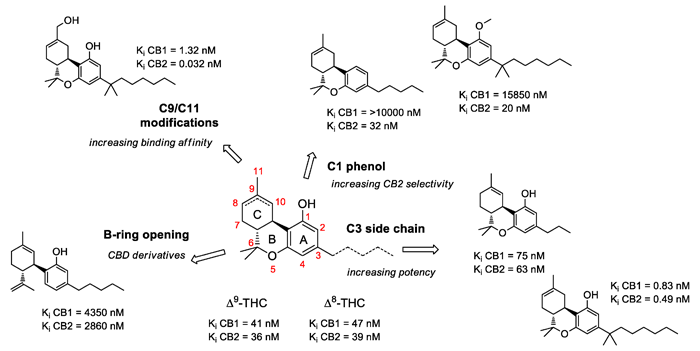
Figure 2. Chemical modifications to the original scaffold of Δ9-tetrahydrocannabinol. Figure from Prandi et al., 2018.
C3, side chain: One of the chemical groups with the greatest variability is the aliphatic chain, which is that zigzag-shaped line that hangs from carbon 3 and that, in the case of THC and CBD, has five points, which represents five carbon atoms. Hence its name, pentyl, which comes from the Greek "penta". Among the natural cannabinoids we find tetrahydrocannabivarin (THCV) and cannabidivarin (CBDV), which are analogs of THC and CBD, but with a shorter chain, of three carbons (figure 2). In 2020, an Italian group led by Giuseppe Cannazza identified four new compounds, structural analogues of THC and CBD, but with aliphatic chains of six and seven carbons respectively, called tetrahydrocannabihexol (THCH), cannabidihexol (CBDH), tetrahydrocannabiphorol (THCP) and cannabidiphorol (CBDP).4 Although the news of a new cannabinoid much more potent than THC was echoed in various specialized media, it was not surprising to those familiar with the structure-activity relationship studies carried out with THC to date, in which the dimethylheptyl substitution had turned out to be render the most potent analogs (Figure 2). Apparently, when we talk about aliphatic chains and cannabinoid receptors, size does matter.
C9, the active carbon: The C9 position in the THC structure presents a methyl group (C11 in the figure). When our body metabolizes THC after consuming it, especially orally, the liver uses some of its CYP 450 enzymes to transform this methyl group (-CH3) into a hydroxyl group (-CH2OH) giving rise to 11-OH-THC and, later, into a carboxyl group (-COOH) originating 11-COOH-THC, which is the metabolite found in urine and which causes a positive result in a drug test. From the point of view of medical chemistry, the hydroxyl and carboxyl residues are way more interesting than the methyl group because they contain oxygen and hydrogen atoms, which normally give rise to a type of inter-molecular interactions called "hydrogen bonds". Hydrogen bonds are essential in nature and are responsible for many natural phenomena, such as the crystallization of water in ice or the folding of DNA. In fact, it is no coincidence that the two semi-synthetic derivatives of THC that have reached further clinical development, nabilone and ajulemic acid (also known as HU-239), have a dimethyl heptyl residue in their aliphatic chain and a polar group in their C9 carbon, a ketone, and a carboxylic acid, respectively.5
C2, the carbon of the acids. As we mentioned at the beginning, the most abundant natural cannabinoids are THCA and CBDA, which are the acidic precursors of THC and CBD, and the naturally occurring form in which the plant produces these molecules. They differ from the neutral form by a carboxyl group in the C2 position, which will be lost in the "decarboxylation" process during combustion, baking or extraction of cannabis buds. Despite being very interesting molecules from a pharmacological point of view, their natural tendency to lose this carboxyl group makes them too unstable to meet the quality standards that govern pharmaceutical developments. In an attempt to stabilize this carboxyl group, Professor Raphael Mechoulam proposed a new semisynthetic, HU-580, which has already been publicized as his latest discovery (and it may be, since Raphi has just turned 90) and promises to be more powerful than THC and CBD.6 Let's see what it is about.
HU-580 maintains the structure of CBDA, but instead of the carboxyl group (-COOH) at the C2 position, it presents a methyl ester (-COOCH3). HU-580 has been tested with some success by regular collaborators of prof. Mechoulam, such as cannabinoid research legends Roger Pertwee and Linda Parker, in animal models of nausea and anxiety.7 From a pharmacological point of view, it would appear that HU-580 is more similar to CBD than to CBDA, which presents additional activities such as, among others, inhibiting the COX enzyme similarly to aspirin and other NSAIDs. And, from the point of view of its pharmacological activity, comparing a carboxyl group with a methyl ester is like comparing a steak knife with a teaspoon: neither pricks nor cuts. Precisely because it lost the ability to create hydrogen bonds. It is like having a plutonium bar, vibrating, reactive, unstable and, to stabilize it, dumping a truckload of concrete on top of it. The result will definitely be more stable, but it can no longer be used to generate energy, destroy the planet, or travel back to the future. In order to stabilize CBDA, it had to be killed.
I hope I am wrong and HU-580 can demonstrate some added value to CBD pharmacology. Otherwise, its career will be short. Both nabilone and ajulemic acid have had mixed luck in their clinical development, and their acceptance and use by the medical community is minimal compared to that of THC. Despite the possible theoretical benefits of semi-synthetics from a pharmaceutical, legal, and intellectual property point of view the truth is that they have not yet managed to unseat the natural cannabinoids that continue to be the best tools that we have in the clinic to modulate the endocannabinoid system.
1. A history of aspirin - The Pharmaceutical Journal. https://pharmaceutical-journal.com/article/infographics/a-history-of-aspirin.
2. Adams, A. J. et al. "Zombie" Outbreak Caused by the Synthetic Cannabinoid AMB-FUBINACA in New York. N. Engl. J. Med. 376, 235–242 (2017).
3. Prandi, C., Blangetti, M., Namdar, D. & Koltai, H. Structure-activity relationship of cannabis derived compounds for the treatment of neuronal activity-related diseases. Molecules vol. 23 1526 (2018).
4. Linciano, P. et al. Identification of a new cannabidiol n-hexyl homolog in a medicinal cannabis variety with an antinociceptive activity in mice: cannabidihexol. Sci. Rep. 10, 1–11 (2020).
5. Burstein, S. H. & Zurier, R. B. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS Journal vol. 11 109–119 (2009).
6. More Potent Than CBD, THC: Dr. Raphael Mechoulam Explains His Latest Discovery. https://www.forbes.com/sites/javierhasse/2020/07/12/dr-mechoulam/?sh=38df958b6a45.
7. Roger Pertwee, C. G. et al. Cannabidiolic acid methyl ester, a stable synthetic analogue of cannabidiolic acid, can produce 5-HT 1A receptor-mediated suppression of nausea and anxiety in rats. Br. J. Pharmacol. 175, 100 (2018).


