By Ismael Galve-Roperh

Ismael Galve-Roperh, Biochemist and molecular biologist with over 20 years experience in cannabinoid research. He has made some major contributions, including discovery of the anti-tumoral role of cannabinoid signalling, its neuroprotective effects in neurodegenerative diseases and the impact of these compounds on neurodevelopment.
Ismael Galve-Roperh1, Alline Campos2, Francisco Guimaraes2, Manuel Guzmán1
1 School of Biology and Instituto Universitario Investigación Neuroquímica, Complutense University, 28040 Madrid (Spain), Centro Investigación Biomédica Red Enfermedades Neurodegenerativas (CIBERNED) and Instituto Ramón y Cajal de Investigaciones Sanitarias (IRYCIS);
2 Dept of Neuropharmacology, University of São Paulo, Riberao preto, Brazil
Recently, the first publically-funded Latin American cannabinoid research network (CannaLatan, www.cyted.org/es/cannalatan), formed by academic investigators and pharma companies of different countries, was launched. CannaLatan aims to promote and boost the results obtained by the different partners of the consortium developing collaborative research and educational projects. The CannaLatan network is devoted to tackle the potential therapeutic applications of under-investigated phytocannabinoids and novel cannabinoid-based molecules, as well to clarify their undesired actions. Here we discuss one of the ongoing projects initiated by CannaLatan with the financial support of Fundación Canna (www.fundacion-canna.es/) , in which we address the anti-psychotic and cognitive actions of acidic forms of phytocannabinoids, namely THCa (Figure 1A).
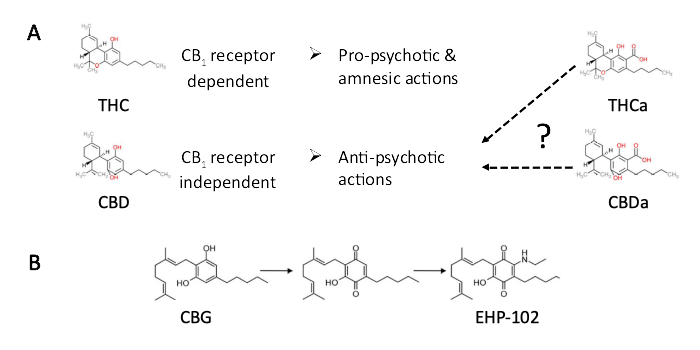
Figure legend. A, Differential actions of the neutral and acidic forms of the two most investigated phytocannabinoids THC and CBD. B, Using phytocannabinoid structural templates for developing improved therapeutic molecules, as exemplified with CBG quinone derivatives.
The plant Cannabis sativa contains more than 160 molecules with a great diversity of chemical modifications based on a common scaffold structure (isoprenylated resorcinyl polyketide core) and known as phytocannabinoids (Hanuš et al., 2016). THC (delta-9- tetrahydrocannabinol) and CBD (cannabidiol) being by far the molecules that have been mostly investigated. The interest of the scientific community during the 20th century on the mechanism of action of THC was mostly propelled by anti-drug institutions, which aimed to demonstrate and disseminate its detrimental actions. In this regard, whereas THC use, like any other bioactive molecule, is not devoid of undesired actions (Ferland and Hurd, 2020), the scientists slowly embraced and investigated the wide variety of potential beneficial applications of THC known by humankind for millennia. THC is a psychoactive molecule that regulates neuronal function primarily via CB1 cannabinoid receptors, which were identified and cloned in the 1990s. In a different manner, the interest in CBD is much more recent. CBD was neglected by scientists as a "non-active" molecule, being one of the main reasons for this lack of interest, the absence of a receptor-mediated mechanism of action. Funny enough, CBD ostracism has lately turned into the preferred cannabinoid molecule as: i) it is a non-psychotomimetic compound (it does not induce psychosis, that is characteristic of CB1 receptor-targeting molecules such as THC; ii) CBD exerts anticonvulsive actions in various refractory epilepsy diseases, and this led FDA and EMA to approve its use as a medicament; and iii) the lack of pro-psychotic effects and cognitive impairment of CBD spares it from most of the access restrictions that apply to THC. Hence, CBD is a safe and very well tolerated compound that has exploded as the "gold" Cannabis molecule and has become the preferred compound for commercial marketing, now being added to many human consumption products and edibles (health-benefiting claimed products, nutritional supplements, and any other thing you can imagine). The efficacy of most CBD-containing products, not of pharmaceutical degree, with low or undetermined CBD concentrations, absence of bioavailability and pharmacodynamic data can be questioned in many cases. However, CBD is an interesting molecule for its purported therapeutic uses. Among other therapeutic actions (Fernández-Ruiz et al., 2020), CBD exerts an anticonvulsant action in epilepsy and relieves various neuropsychiatric disorders symptoms. In this regard, CBD has been repeatedly demonstrated to exert anxiolytic, antidepressive and antipsychotic actions in preclinical models of various pathological conditions (Fig. 1A). There is moderate evidence that CBD alleviates symptoms of schizophrenia, social anxiety disorder, comorbidities of autism spectrum disorder, and attention deficit hyperactivity disorder in humans (Crippa et al., 2020). Last, but not least, CBD is being explored for the management of non-motor symptoms of neurodegenerative diseases (i.e., Parkinson's disease and Alzheimer's disease), to mention some examples.
In addition to the acute modulation of behavioral aspects, cannabinoids can also influence neural cell fate, controlling cell survival and death signalling pathways. THC can exert pro-survival actions of neurons in neurodegenerative diseases and counteract cell death induced by acute neuronal insults (excitotoxicity, hypoxic-ischemic damage, traumatic brain injury and others)(Di Marzo et al., 2015). Of interest, THC, by acting on CB1 receptors located in neural progenitor cells, and CBD by acting through still unclear mechanisms, promote the generation of newborn neurons in the adult mouse brain (Diaz-Alonso et al., 2012). This process, known as "adult neurogenesis", takes place in discrete brain areas (hippocampus and subventricular zone) of rodents, and its existence and relevance in the human brain is the object of controversy and debate. Overall, the THC-CBD "duet" exert complementary beneficial actions in pathological states of the nervous system. However, the mechanism of action of CBD remains largely unclear, though it has been shown to modulate several receptors and signalling pathways. In any case, it is important to keep in mind that, at least to date, most demonstrated therapeutic actions of cannabinoid-based products are conceivably due to their THC content (www.fundacion-canna.es/cannabis-vs-thc-son-realmente-tan-distintos).
Besides THC and CBD, a new wave/trend of research aims to address the physiological impact of other minor cannabinoids present in the plant [i.e., THCa (tetrahydrocannabinolic acid), CBDa (cannabidiolic acid), cannabigerolic acid (CBGa), tetrahydrocannabivarin (THCV), cannabidivarin (CBDV), cannabichromene (CBC) and its acid form CBCa and others](Stone et al., 2020). Phytocannabinoids are produced by complex metabolic pathways, and cannabigerol (CBG) is considered the common precursor for most of them. Typically, THCa and other acidic forms of cannabinoids are converted into their neutral bioactive molecule forms by heat-induced decarboxylation. Efforts to elucidate whether such acid forms of cannabinoids could be of therapeutic interest, as so far, they are considered devoid of undesired psychoactive effects, are ongoing. Emerging new data suggest that CBG, CBDV and THCV, as well as THCa and CBDa, might be helpful in treating some symptoms of neurological and psychiatric diseases (Fig. 1A). Although available evidence is still weak and requires further investigation, THCa is neuroprotective in mice by acting via the nuclear receptors PPARγ and has been suggested to exert immunomodulatory actions. It also attenuates adiposity and metabolic disease caused by diet-induced obesity.
Using the preclinical model of NMDA glutamate receptor antagonism, the University of Sao Paulo (Brazil) and Complutense University (Spain) groups are evaluating the putative antipsychotic and anxiolytic actions of THCa. THCa administration to rodents rescued social interaction and cognitive function deficits, evaluated by the novel object recognition test. Importantly, THCa was as effective as the typical antipsychotic drug clozapine. Ongoing research focuses on determining the molecular mechanism of action for THCa, which may involve the regulation of PPARγ, or the formation of CB1 receptor heteromers, similarly to other phytocannabinoids such as CBD and CBG. Regarding other acidic cannabinoid counterparts, CBDa is neuroprotective, although by acting through different mechanisms than CBD. Also, CBDa prevents emesis and vomiting, being more potent than its neutral CBD form. The antinociceptive action of CB1 receptor-targeting cannabinoids is also observed with CBDa. CBGa has been shown to induce either anticonvulsive or proconvulsive actions in different preclinical models of epilepsy.
Unfortunately, the field of cannabinoid therapeutic applications is limited by the requirement of promoting economic benefits to the industry which stands behind. Some companies develop their financial benefit strategies by intellectual property protection of methods for cannabinoid delivery, isolation, and purification. Others have chosen to design new molecule structures by chemical modification of phytocannabinoids to improve their therapeutic actions, ameliorate their bioavailability, and attenuate their undesired effects (Fig. 1B). Among others, CBD and CBG derivatives, like the EHP-101 and EHP-102 compounds, respectively, have been developed, leading to the identification of molecules of interest that fulfill some of these criteria. The EHP compounds have been actively investigated for their therapeutic actions against neurodegenerative and neuroinflammatory disease consequences. Preclinical research succeeded to identify that CBG derivative EHP-102, by acting via PPARγ is neuroprotective in Huntington's disease mouse models. The neuroprotective effect of EHP-102 adds to its proneurogenic action, hence increasing the generation of subventricular zone-derived neurons that migrate towards the degenerating striatum. Of note, this molecule is effective by oral administration and, as a partial agonist of the receptor, does not induce the characteristic detrimental effects of PPARγ full agonists. Another promising drug, HU-580, based on the methyl ester modification of CBDa, is under investigation for its anti-nausea and anxiolytic actions.
In summary, research on the potential therapeutic actions of cannabinoids has experienced an exponential growth in the last decades and has succeeded to identify and demonstrate interesting beneficial effects for THC and CBD. Whereas more basic and sound research, and mostly, standardized clinical trials are still needed to advance in the translation of preclinical research from the bench to bedside for these molecules, new avenues are actively explored to identify if other, minor phytocannabinoids are of interest for biomedical purposes. We predict that, in the future, the cannabinoid field will benefit from the diversity of plant-derived molecules and will demonstrate novel applications for the management of nervous system disorders and their symptoms.
Acknowledgements: Cyted funding of CannaLatan network, Fundación Canna for their support for THCa research, and Phytoplant Research (Spain) for kind donation of purified phytocannabinoids.
Selected references
Crippa JA, de Lima Osório F, Hallak J, Guimarães FS, Zuardi AW. Cannabinoids for the treatment of mental disorders. The Lancet Psychiatry 2020; 7: 125–126.
Diaz-Alonso J, Guzman M, Galve-Roperh I, Díaz-Alonso J, Guzmán M, Galve-Roperh I. Endocannabinoids via CB₁ receptors act as neurogenic niche cues during cortical development. Philos Trans R Soc Lond B Biol Sci 2012; 367: 3229-41.
Ferland JMN, Hurd YL. Deconstructing the neurobiology of cannabis use disorder. Nat Neurosci 2020; 23: 600-610.
Fernández-Ruiz J, Galve-Roperh I, Sagredo O, Guzmán M. Possible therapeutic applications of cannabis in the neuropsychopharmacology field. Eur Neuropsychopharmacol 2020: 1-18.
Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: A unified critical inventory. 2016
Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 2015; 16: 30-42.
Stone NL, Murphy AJ, England TJ, O'Sullivan SE. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br J Pharmacol 2020; 177: 4330-4352.


