By Francisco Molina-Holgado / Eduardo Molina-Holgado

Dr Francisco (Paco) Molina-Holgado experience includes a Readership in Neuroscience in London, followed by the leadership of multidisciplinary groups of academic staff and technicians in the areas of biomedicine and neuroscience, with over 20 years of experience in the academia and pharmaceutical industry. He has been the Head of the Biomedical Science programme at University of Roehampton in London. At present, he is the Head of the Neural Stem Cell Laboratory at Roehampton. The main interest of his laboratory is the role of the brain endocannabinoid system in brain repair, and in particular research in neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease and Multiple Sclerosis. He is a regular speaker in different neuroscience areas (including mental health, ageing and drug abuse), and supervisor of MSc projects and PhD Thesis at several Higher Education institutions. He is a founding member and former VP of Spanish Researchers in UK (SRUK). Dr Molina-Holgado also has an interest in drug discovery and translational aspects of neuroscience, having contributed to the development and patent of new chemical entities of relevance in neurodegenerative disease. Dr Molina-Holgado sits on a number of scientific committees and acted as an editorial peer reviewer, external examiner, and grant reviewer for different UK, USA, and EU universities, founding bodies and charities.
Eduardo Molina-Holgado

In 1989 after graduating in biological sciences (specialty of biochemistry and molecular biology) moved to the Université de Montreal in Canada, where he held a doctoral thesis in neurological sciences at the Faculty of Medicine. After the presentation of his thesis he joined the laboratory at the Department of Pharmacology and Therapeutics (McGill University, Montreal, Canada) to investigate the role of neurotransmitter receptors in oligodendrocytes of the nervous system. Back in Spain in 1997 he joined the group of Dr. Carmen Guaza of the Cajal Institute of the Higher Council of Scientific Research (CSIC). Among other lines of work could provide data demonstrating that endocannabinoids have important effects on the biology of glial cells under normal conditions and models of injury. Since 2003 he directs a research team at the National Hospital of Paraplegics in Toledo where he investigates the role of the endocannabinoid system as a therapeutic target after a traumatic spinal cord injury.
Neuroprotective and neurogenic effects of the brain endocannabinoid system
Maintenance of homeostasis is an essential requirement for the survival of all living beings. The immune system (IS) and central nervous system (CNS) are particularly important for restoring physiological balance in the organism. For a variety of reasons, in neurodegenerative diseases this homeostatic balance is lost. Some of these causes are known and others are not, which can make treating such pathologies tremendously difficult. Among the best-studied causes of the onset of the neurodegenerative process are the appearance of free radicals (derived from oxygen or nitrogen); excitotoxicity; protein aggregation (Parkinson's disease and Alzheimer's disease); autoimmunity (multiple sclerosis); hypoxia (ictus); neuroinflammation and mitochondrial dysfunction with the associated creation of a pro-inflammatory scenario, neurotoxicity and finally neuron death.
Throughout the evolutionary process, the central nervous system in mammals has developed a range of endogenous repair strategies in response to brain damage, trauma or neurodegeneration. Among other mechanisms, the brain endocannabinoid system, initially considered as a neuromodulatory system, has been shown to be of key importance in these neuroregenerative processes due to its neuroprotective (anti-oxidant and anti-inflammatory) and neurogenic (production of new nerve cells in the brain) effects in neurodegenerative diseases (Molina-Holgado & Molina-Holgado, 2010).
The brain endocannabinoid system is made up of cannabinoid receptors (CB1 and CB2), endogenous ligands or endocannabinoids (arachidonylethanolamide or anandamide and 2-arachidonoylglycerol ( 2-AG)) and the enzymatic machinery necessary for the synthesis (NAPE and DAGL) and degradation (FAAH, MAG) of these endogenous ligands (Pertwee et al, 2010). It is important to note that in the human brain, the CB1 cannabinoid receptor is the most abundant of the various receptors binding to G proteins (Joy et al., 1999), with far larger numbers than that of receptors considered as classic neurotransmitters (dopamine, adrenaline, serotonin, acetylcholine and opioids). In this context, the notable presence of cannabinoid signalling mechanisms in the brain logically implies a special relevance in cell function and communication processes.
Over the last two decades, several research groups have demonstrated that two-way communication between neuroimmune signalling networks and the brain endocannabinoid system is of key importance due to their neuroprotective and neurogenic effects in response to neurodegenerative processes, suppressing or attenuating chronic neuroinflammatory responses, regulating the release of pro-inflammatory mediators or activating processes of adult neurogenesis (Molina-Holgado & Molina-Holgado, 2010). For all of these reasons, the brain cannabinoid system is now viewed as a therapeutic target of enormous potential due to its neuroprotective effects in pathological processes associated with brain damage, ischemia and chronic neurodegenerative diseases.
Alzheimer's disease
Alzheimer's disease is characterised by a progressive cognitive deterioration. It is the most common form of dementia (accounting for 60% of all cases) and affects a population of 33 million people worldwide (Wisniewski & Goñi, 2014; Graham et al, 2017).
The molecular mechanisms underlying this pathology are not clearly described, making treatment difficult. Nonetheless, the aetiology of Alzheimer's disease is associated with an accumulation of the protein beta amyloid (βA) in the brain in the form of dimers, trimers and oligomers that cause damage to the synaptic structures, alterations to processes of adult neurogenesis and ultimately, neuron death (Crews et al. 2009). Figure 1 shows the neurotoxic effect of the human protein βA on cortical neurons that results in neuron death. Among the cell signalling pathways involved in the deregulation of adult neurogenesis are Notch, CDK5, Wnt/BMP and Ca2+ flux (Crews et al. 2009). In contrast, several members of the disintegrin and metalloproteinase (ADAM) family which increase the levels of the precursor of the protein amyloid, reduce the formation of amyloid protein, their activity being associated with an increase in pro-neurogenic activity and axonal growth (Yang et al. 2005). Proteins from the ADAM family are particularly important for the maturing and processing of proteins such as Notch, the precursor of the protein amyloid (APP) and the growth factor TGF-alpha. Likewise, protein-protein interactions can be established with integrins. Other studies confirm that in pathological situations or in experimental models of neurogenesis, the activity of ADAM10, the TNF-α converting enzyme (TACE/ADAM17) and ADAM21 induce proliferation of neural precursors (Yang et al. 2005; Katakowski et al. 2007; Rubio-Araiz et al. 2008).
Therapeutic potential of endocannabinoid system in Alzheimer's disease
Fifty years ago, the discovery of the psychoactive principle of Cannabis sativa L. plants, Δ9-tetrahydrocannabinol (Δ9-THC), marked the beginning of research into the physiological role of cannabinoids (Mechoulam & Gaoni, 1965). In addition to their well-studied effects on behaviour, cannabinoids also play an important neuroregulatory in the CNS.
The endogenous brain cannabinoid system has recently drawn the attention of numerous research groups in the area of Alzheimer's disease because of the variety of physiological processes it either regulates or in which it is involved, inter alia: neuroinflammation, neurogenesis, brain connectivity, oxidative stress, glial activation, elimination of cellular remains or cellular elements and macromolecules (Marsicano et al., 2003; Molina-Holgado & Molina-Holgado, 2010; Bilkei-Gorzo, 2012). Two areas of the brain have been the subject of detailed studies on the influence of the cannabinoid system in Alzheimer's disease, the hippocampus (the area that controls memory and learning processes) and the cortex (in evolutionary terms, the most modern area of the human brain) (Graham et al. 2017). As consequence of the neurotoxicity induced by the proteins βA, a series of cellular events are caused which involve activation of the brain endocannabinoid system, whereby it attempts to block the development of the neurodegenerative process and ultimately neuron death.
In microglia cells (the brain's resident macrophages) in contact with the oligomers (senile plaques) of the proteins βA, there is a significant increase in the expression of the cannabinoid receptor CB2 (Benito et al. 2003; Ramirez et al. 2005; Aso and Ferrer, 2016) in an attempt to offset the neurodegenerative processes that have begun. It is worth noting that in experimental models of Alzheimer's disease, the pathogenic role of microRNA miR-139 has recently been described in modulating the neuroprotective and anti-inflammatory activity of the receptor CB2 in the hippocampus (Tang et al., 2017). At the same time, as a consequence of the neuron death associated with the progression of Alzheimer's disease, significant reductions have been described in the expression of the CB1 receptor in the hippocampus and in the basal ganglia (Ramirez et al. 2005). These changes in the expression levels of cannabinoid receptors mean that it may be possible to evaluate their expression as potential biomarkers of the disease.
When the expression of these CB1 and CB2 receptors is modified, changes are also produced in the levels of endogenous cannabinoid ligands.
Several research groups have described a significant increase in cannabinoid tone through an increase in the synthesis and release of endocannabinoids. Particularly relevant are the increase in the levels of 2-AG (the most abundant endocannabinoid in the CNS) in the area of the hippocampus in response to the appearance of neurodegenerative processes and gliosis (Van der Stelt et al. 2006). With regard to the enzymatic machinery that regulates the synthesis (DAGL) and degradation (MAGL) of the 2-AG, the changes in the levels of expression of these enzymes are very relevant, since the DAGL/MAGL axis modulates the increase of neuroinflammation associated with the disease. (Nomura et al, 2011; Chen et al, 2012; Zhang et al., 2014). At the same time, in astrocytes associated with senile plaques, increases have been described in the expression of FAAH (an enzyme that degrades the anandamide), causing an increase in arachidonic acid, brain prostaglandins and an increase in the neuroinflammation associated with the disease (Nomura et al, 2011).
If we analyse the neurodegenerative/neuroinflammatory process that occurs in Alzheimer's disease, the reduction of the expression of CB1 cannabinoid receptors and the increase in the hydrolysis enzymes (FAAH) corresponds to the progression of the disease and the increase in neuroinflammation (Fernandez-Ruiz et al, 205). On the contrary, the increase in signalling via CB2 receptor and increase in the endogenous ligand 2-AG would define a reparative or neuroprotective endogenous response (Benito et al. 2003; Ramirez et al. 2005).
In order to understand the neuroprotective and anti-inflammatory effect developed by the endocannabinoid system, it is important to note its role as an intercommunicator of the immune system and CNS (Molina-Holgado & Molina-Holgado, 2010) in both physiological and pathological situations. Different neuroimmune networks are regulated by cannabinoid mediators, and this is of therapeutic interest in Alzheimer's disease. The existence of a two-way communication has recently been described between the expression of endocannabinoids (AEA and 2-AG) and anti-inflammatory and neuroprotective mediators as the antagonist of the interleukin-1 receptor (IL-1RA) (Molina-Holgado & Molina-Holgado 2010; Garcia-Ovejero et al, 2013). This two-way communication is of therapeutic interest in Alzheimer's disease, since by increasing the endocannabinoid tone, one would at the same time increase the release of anti-inflammatory and neurogenic mediators, which would have positive effects in blocking the progression of Alzheimer's disease.
To conclude, one should mention various pre-clinical in vitro and in vivo studies on the pathology of Alzheimer's disease which have demonstrated the therapeutic potential of cannabidiol (CBD) and combinations of CBD-THC (Watt and Karl, 2017). These promising preliminary results, obtained in experimental models of Alzheimer's disease, require description of the neuroprotective mechanisms, probably based on the experimental data described above, and of course their definitive confirmation in clinical trials.
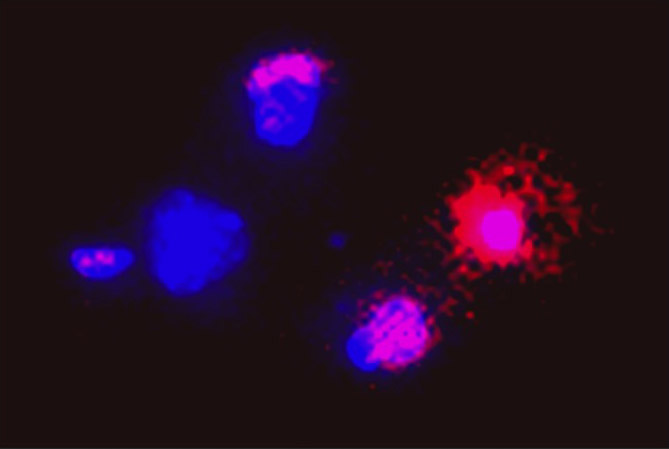
Figure 1. The human protein β-amyloid, which causes Alzheimer’s disease, causes the death of cortical neurons. Neuron death was evaluated with a double marker fluorescence test using propidium iodide (red) and bisBenzimide H 33342 (blue). The microphotograph shows necrotic neurons and viable neurons (blue) in response to neurotoxicity induced by β-amyloid (3µM, t=24h) (F. Molina Holgado, 2017)
Bibliography
Aso E, Ferrer I. (2016) CB2 Cannabinoid Receptor As Potential Target against Alzheimer's Disease. Front Neurosci. 10:243.
Benito C, Nuñez E, Tolon RM et al (2003) Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci 23: 11136–11141.
Bilkei-Gorzo A. (2012) The endocannabinoid system in normal and pathological brain ageing. Philos Trans R Soc Lond B Biol Sci. 367(1607):3326-41.
Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, Teng Z, Chen C. (2012) Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease. Cell Rep. 2(5):1329-39.
Crews L, Tsigelny I, Hashimoto M, Masliah E. (2009) Role of synucleins in Alzheimer's disease. Neurotox Res. 16(3):306-17.
Fernandez-Ruiz J, Romero J, Ramos JA. (2015) Endocannabinoids and Neurodegenerative Disorders: Parkinson's Disease, Huntington's Chorea, Alzheimer's Disease, and Others. Handb Exp Pharmacol. 231:233-59
Garcia-Ovejero D, Arevalo-Martin A, Navarro-Galve B, Pinteaux E, Molina-Holgado E, Molina-Holgado F. (2013) Neuroimmmune interactions of cannabinoids in neurogenesis: focus on interleukin-1β (IL-1β) signalling. Biochem Soc Trans. 41(6):1577-82.
Graham WV, Bonito-Oliva A, Sakmar TP. (2017) Update on Alzheimer's Disease Therapy and Prevention Strategies. Annu Rev Med. 68:413-430.
Joy JE, Watson SJ, Benson JA eds. Marijuana and medicine. Washington, DC, USA: National Academy Press, 1999.
Katakowski M, Chen J, Zhang ZG, Santra M, Wang Y and Chopp M. (2007) Stroke-induced subventricular zone proliferation is promoted by tumor necrosis factor-alphaconverting enzyme pro- tease activity. J. Cereb. Blood Flow Metab. 27, 669–678.
Marsicano G., Goodenough S., Monory K. et al. (2003) CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88.
Mechoulam R, Gaoni Y. (1965) A total synthesis of dl-delta-1-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc. 87:3273-5.
Molina-Holgado E, Molina-Holgado F. (2010) Mending the broken brain: neuroimmune interactions in neurogenesis. J Neurochem. 114(5): 1277-90.
Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. (2011) Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334(6057): 809-13.
Pertwee, R. G., Howlett, A. C., Abood, M. E., Alexander, S. P., Di Marzo, V., Elphick, MR, Ross, RA. (2010). International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev, 62(4), 588-631.
Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML (2005) Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci 25: 1904–1913.
Rubio-Araiz A, Arevalo-Martin A, Gomez-Torres O, Navarro-Galve B, Garcia-Ovejero D, Suetterlin P, Sanchez-Heras E, Molina- Holgado E and Molina-Holgado F (2008) The endocannabinoid system modulates a transient TNF pathway that induces neural stem cell proliferation. Mol. Cell. Neurosci. 38, 374–380.
Tang Y, Bao JS, Su JH, Huang W. (2017) MicroRNA-139 modulates Alzheimer's-associated pathogenesis in SAMP8 mice by targeting cannabinoid receptor type 2. Genet Mol Res. 16(1).
Watt G, Karl T. (2017) In vivo Evidence for Therapeutic Properties of Cannabidiol (CBD) for Alzheimer's Disease. Front Pharmacol. 8:20. eCollection 2017.
van der Stelt M, Mazzola C, Esposito G et al (2006) Endocannabinoids and β-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci 63:1410–1424.
Wisniewski T, Goñi F. (2014) Immunotherapy for Alzheimer's disease. Biochem Pharmacol. 88(4): 499-507
Yang P, Baker KA, Hagg T. (2005) A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult rodent CNS. Comp Neurol. 490(2):163-79.
Zhang J, Hu M, Teng Z, Tang YP, Chen C. (2014) Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer's disease. J Neurosci. 34(45):14919-33.