It has been a long road to 2023, but we have learned how to change an ancestral relationship with Cannabis sativa L., to an articulated, documented science.
Since the first cannabinoids were isolated in the '40s, (1) we moved towards slow and steady progress.
First, the determination of the structure of THC and CBD in the '70s (2)(3)(4), and then the discovery and codification of the endocannabinoid system in the late '80s, which was perfected in the early '90s (5)(6). Nowadays, we are definitively quitting the dark years of governmental prohibition and entering an era of transparency and knowledge.
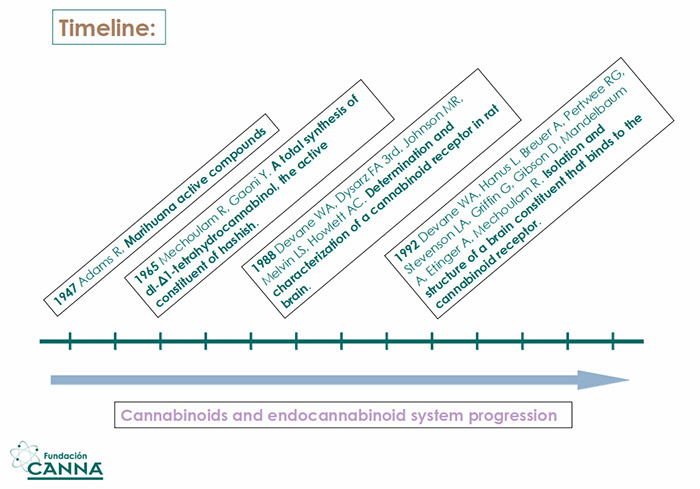
Fig.1 Timeline of relevant progress in the understanding of cannabinoids
Today, we can understand the cannabinoid system in great detail.
We know the structure, the distribution and, partially, the role of CB1 and CB2 receptors, (7) and we can produce and test an interminable number of compounds capable of binding and/or activating them.
Synthetic cannabinoids and cannabimimetic compounds have been extensively tested in the last 15 years and their toxicity reported all over the world.(8)
You may remember the case of ¨Spice¨ and ¨K2¨: herbal blends commercialized in the EU and the USA in the late '00s. The mixture found in Spice, which did not include Cannabis Sativa L. was doped with a wide array of synthetic compounds like CP 47,497, HU-210 and JWH-018.
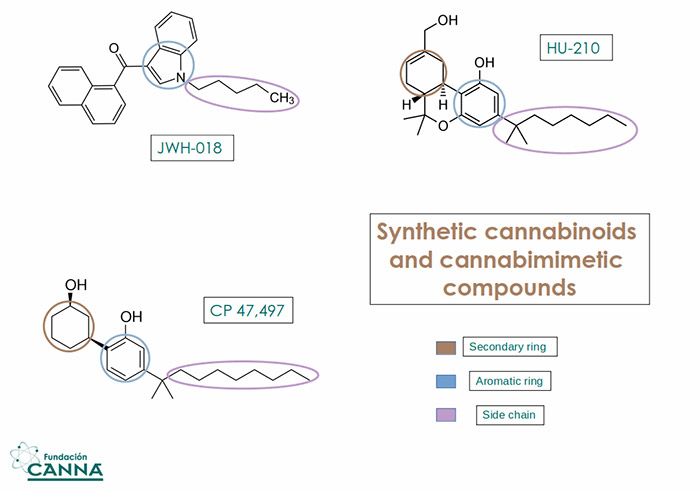
Fig.2 Some of the psychoactive compounds isolated in Spice and other synthetic herbal blends
These compounds have qualities that surpass that of THC and can generate long-lasting psychedelic effects. The consumption of Spice-like mixes generates nasty addictions, worse than the common THC-drawback, with persistent psychiatric and nervous issues. (9)
Thus, between 2009 and 2012, most EU countries and the USA approved laws to limit or ban synthetic cannabinoids and, nowadays, Spice-like blends are confined to digital markets.
The ban on Spice has highlighted a weakness in the international system: cannabinoids may be banned one by one, but it is almost impossible to generalize their structures (there are at least 5 groups of cannabimimetic compounds) and prevent the synthesis of new compounds that interact with CB1 and CB2.
Meanwhile, the markets evolved but, in 2022, the situation started repeating itself.
WHAT IS HHC?
HHC-hexahydrocannabinol is a rare fitocannabinoid, isolated and characterized in the first studies in the '40s (1).
Alongside the effort to extract and concentrate HHC in cannabinoid extracts, HHC is a product of direct hydrogenation of THC isomers (Δ8-Δ9-Δ10) that converge to a single racemic mix.
The difference between HHC and THC in terms of the basic formula is minimal, but the addition of one hydrogen molecule (2 atoms) removes the unsaturation on the secondary ring, allowing a more flexible structure.
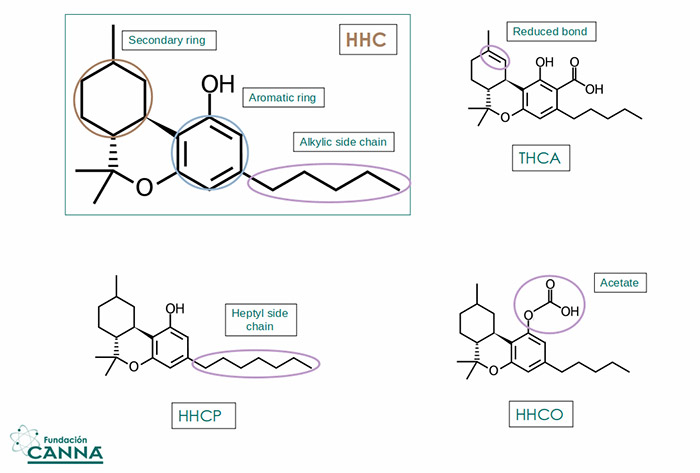
Fig.3 HHC and some common derivatives compared with THCA
The hydrogenation itself has been coded in numerous patents and papers (10)(11) and is based on the catalytic properties of electron-rich metals, capable of reversible red/ox additions and eliminations.
In the right conditions, compounds of Ni, Pt, Pd etc., can break the H-H bond of the dihydrogen molecule (oxidative addition), bind to the substrate (in our case a THC isomer), transfer the hydrogen atoms on the bound substrate, and detach via reductive elimination.
Depending on the purity of the reaction mix (we imagine a THC isomer mix) and the selection of the specific catalyst (PtO2 and Pt/C), the reaction proceeds, cleanly generating a racemic mix of enantiomers: 9R and 9S HHC.
9R-HHC and 9S-HHC have the exact same basic structure, differing just for the 3D orientation (conformation)of a methyl group on the secondary ring.
The absence of the double bond typical of THC increases the stability of HHC towards UV and oxidative reactions, increasing shelf times and permitting more aggressive purification treatments.
HHC-labeled samples, analyzed all over the world, were reported to contain not only HHC enantiomers but also a few HHC derivatives and alternatives like HHCA hexahydrocannabinol acid (the same as THCA for THC), HHCO hexahydrocannabinol acetate (the same as THCO for THC) and other compounds that differ in the length of the alkylic chain on the core aromatic ring like HHCP hexahydrocannabiphorol (7 member alkylic chain instead of 5).
HHC AND THE ENDOCANNABINOID SYSTEM
As can be deduced from the chapters above, HHC can interact with Cb1 and Cb2 receptors and generate psychoactive effects like those generated by Δ9-THC.
If we consider the effects of Δ9-THC as 1, we may say that 9R-HHC has 3/4 of Δ9-THC activity (9S-HHC is less active), Δ8-THC has 1/2 and CBN has 1/10.
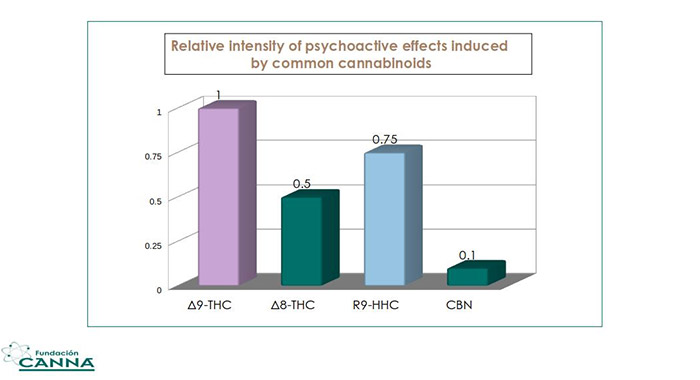
Fig.4 Relative psychoactive effects induced by common cannabinoids
The HHC phenomenon has shifted the attention of the scientific world towards synthetic cannabinoids.
The growth of studies around HHC-like structures has grown to the point of requiring a rapid response from international institutions, culminating with a report from the EU (13) (https://www.emcdda.europa.eu/publications/technical-reports/hhc-and-related-substances_en).
In these pages, the Commission advises against the commercialization of HHC on the free market and for the pharmacological investigation of its medical properties.
The latest reviews (12) show many promising results: HHC is undergoing the same tests that formalized the properties of CBD and THC.
In these tests, the action of HHC is described as closer to that of THC than CBD, including anti-tumor activity and analgesic properties.
Conversely, HHC is also reported for several adverse effects (9) representing a mix of those associated with fitocannabinoids and synthetics:
- Anxiety
- Sleepiness
- Dry mouth
- Increased appetite
- Fear
- Fast heartbeat
- Red eyes
and severe ones, connected with heavy abuse of cannabinoids in general, like:
- Stroke
- Seizure
- Heart attack
- Breakdown of muscle tissue
- Kidney damage
- Psychosis
- Severe vomiting
HHC AND THE MARKET
The recent spike in awareness of cannabinoids has not been reflected in a cautious reaction on the part of the producers and resellers that have invaded the market with a plethora of HHC, HHCO, and HHCP products.
HHC is still not illegal in most countries, even those with restrictive rules for THC and CBD.
In Europe, HHC is not scheduled in the EU Green List (List of Psychotropic Substances Under International Control) but, since 2023, HHC has been officially monitored by the European Monitoring Center for Drugs and Drug Addiction that promised a regulation shortly.
In the USA, the legal standing of HHC and other cannabinoids, including Δ8-THC, is unclear.
Many suppliers argue that it is allowed because it occurs naturally, and no regulation prohibits it.
The reality is that naturally-extracted HHC is uncommercial (too scarce), but because of its natural appearance, producers can derive it from other fitocannabinoids like CBD and THC via semi-synthesis (totally synthetic HHC is still prohibited).
While researching this article, we found all kinds of unconfirmed claims for HHC: many were just transcripts from other cannabinoid properties and others were utterly bizarre. One alarmed us, as it sustained – without any proof – that HHC consumption should not analyze as positive in drug tests, introducing a possible risk to public health.
As we are skeptical about this assertion, we want to repeat that the only way to assure a negative result in a drug test is to avoid cannabinoids completely, and the only responsible way to consume any type of cannabinoid is far from any possible risk (and test).
HISTORY REPEATING
After many years of monitoring the legal and illegal markets in Europe, we cannot really sponsor HHC as a realistic alternative to either CBD or THC for recreational use in the countries that ban them. Data is still scarce, and the risk of adverse effects is tangible.
As with what happened with the previous generation of synthetic cannabinoids, the EU will soon pronounce on the topic, and we expect an incisive regulation on HHC, its analogs, and any other cannabimimetic compounds that are already appearing on the horizon, such as THCP and CBDP or THCB and CBDB (7 and 4 member alkylic chain instead of 5).
This upcoming block will not stop any legitimate pharmacological and veterinary interest in this class of compounds, which is growing with incredible rapidity.
Semi-synthetic and synthetic cannabinoids are much more controllable (synthesized and not grown) than THC and CBD and are destined to substitute and surpass both.
BIBLIOGRAPHY:
1. US 2419937, Adams R, "Marihuana active compounds", issued 6 May 1947.
2. Burstein S. Review. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015;23:1377–1385.
3. Mechoulam R, Gaoni Y. A total synthesis of dl-Δ1-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc. 1965;87:3273–3275.
4. Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147:S163–S171.
5. Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988 Nov;34(5):605-13. PMID: 2848184.
6. Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 Dec 18;258(5090):1946-9. doi: 10.1126/science.1470919. PMID: 1470919.
7. https://www.fundacion-canna.es/sistema-endocannabinoide
8. https://www.fundacion-canna.es/cannabinoides-sinteticos
9. Spaderna M, Addy PH, D'Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl). 2013 Aug;228(4):525-40. doi: 10.1007/s00213-013-3188-4. Epub 2013 Jul 9. PMID: 23836028; PMCID: PMC3799955.
10. US 9694040B2, Scialdone MA, "Hydrogenation of cannabis oil", registrada el 10 de noviembre de 2016, asignada a Research Grow Labs.
11. US 10071127B2, Scialdone MA, "Hydrogenation of cannabis oil", registrada el 21 de septiembre de 2017, asignada a Research Grow Labs.
12. Ujváry, I. Hexahydrocannabinol and closely related semi-synthetic cannabinoids: A comprehensive review. Drug Test Anal. 2023; 1- 35. doi:10.1002/dta.3519
13. https://www.emcdda.europa.eu/publications/technical-reports/hhc-and-related-substances_en