By Andrew F Scheyer

Andrew F Scheyer is a post-doctoral fellow at the Institut de Neurobiologie de la Méditerranée in Marseille, France in the laboratory of Dr. Olivier J Manzoni. He specializes in electrophysiology and has a keen passion for scientific communication. Prior to working in his current position, Andrew completed his PhD on the subject of synaptic mechanisms underlying cocaine addiction and withdrawal in 2015 in the laboratory of Marina E Wolf at Rosalind Franklin University in North Chicago, in the United States. Since then, his research has focused on the developmental trajectory of the endocannabinoid system and how it is impacted by prenatal and early postnatal cannabis exposure. His recent publications in Trends in Neurosciences and Biological Psychiatry focus on disturbances in neurological development and early-life behaviors following perinatal cannabis exposure. When he is not in the lab, Andrew is a ultra-distance cyclist and avid gardener.
As the current global reassessment of cannabis use continues to trend towards decriminalization and legalization, an honest appraisal of the risks of cannabis use must advance in tandem. While much discussion has already highlighted the dangers of cannabis use during critical periods of brain development, such as childhood and adolescence, little attention has been paid to cannabis use during pregnancy and the early postnatal period (i.e. during breastfeeding). We know, however, that the active ingredients in cannabis, such as THC and CBD, are transferred to the developing fetus when they are used by women during pregnancy. As with other drugs, this exposure has the potential to significantly impact the developing baby, leaving lasting marks that will alter its life before it has even entered the world.
Currently, 70% of women surveyed in the United States believe that there is "slight or no risk" associated with cannabis use during pregnancy1. The perception of cannabis as a natural alternative to pharmaceutical drugs positions it as a friendlier, seemingly more benign remedy for many of the maladies associated with pregnancy, such as nausea, anxiety, physical discomfort, and restlessness. Moreover, a recent study in the US state of Colorado, where cannabis is both recreationally and medically legalized, found that nearly 70% of cannabis dispensaries recommend the use of cannabis during the first trimester of pregnancy in order to combat nausea amongst these other pregnancy-associated discomforts2. However, both laboratory-based research and studies of humans who have used cannabis during pregnancy have shown that there are significant negative consequences to this use.
Cannabis use during pregnancy carries significant risks due to the involvement of the endocannabinoid system in many crucial developmental processes. Briefly, the endocannabinoid system is a biological system found throughout the body and brain made up of neurotransmitters and receptors that serves to fine-tune nearly every physiological and cognitive function, from heart rate and digestion to complex mental tasks like memory and social behavior. The active ingredients in cannabis function by interacting with this very system, which explains why the effects of cannabis are so widespread throughout the body and brain. The endocannabinoid system is a crucial participant in many important developmental steps, from the fertilization of eggs to the earliest organization of nascent brain regions. When cannabis is used by a pregnant or breastfeeding mother, and its active ingredients are transmitted to the developing fetus or infant, these processes are disrupted, resulting in an array of consequences.
Research into the consequences of cannabis use during pregnancy is conducted via two major avenues: epidemiological studies (those which gather data from human populations) and laboratory research using animal models. Importantly, there is remarkable coherence between studies carried out in rodents and the effects seen in humans, which have been highlighted here in Figure 1. While such agreement between controlled, laboratory modeling of disorders and real world conditions is not universal amongst scientific pursuits, the data which have been produced in rodent models of cannabis use during pregnancy have not only confirmed what is seen in humans exposed to cannabis in the womb, they have also provided significant and valuable insight into the molecular and cellular mechanisms underlying these effects, which are difficult to ascertain with non-invasive techniques in humans. On the other hand, complex cognitive parameters, like social behavior and task-planning, which are difficult to observe in animals, have been robustly researched in the epidemiological studies. Thus, the two arms of research work to complement each other and provide a more thorough picture of the consequences of prenatal cannabis exposure.
Human Studies
On the epidemiological front, three major studies have thus far documented the outcomes of cannabis use during pregnancy on the development of the children of these users. First, the Ottawa Prenatal Prospective Study (OPPS), which was initiated in 1978, looked at low-risk, European-American, middle-class pregnant women and followed their offspring until early adulthood (18-22 years of age)3-6. Elsewhere, the Maternal Health Practices and Child Development study (MHCPD), focused on high-risk pregnant women of mixed ethnicity and low socioeconomic status, and followed their offspring until adolescence (14 years of age)7-11. Finally, the Generation R study, which is ongoing, enrolled pregnant women in middle-to-high socioeconomic status and continues to follow the offspring12-13.
So, what have these epidemiological studies found? In contrast to prenatal alcohol or cigarette exposure, the effects of cannabis use during pregnancy are mostly manifested as behavioral and cognitive problems. Low birth weights have been found in all of these studies, though no other gross abnormalities are noted in the offspring of cannabis-using mothers. Intelligence, as measured by standardized testing such as IQ and early-life benchmarks, is rarely impacted by cannabis exposure in the womb. While the MHPCD reported slow mental development during the first year of, these effects were mostly absent halfway through their second year. Both the MHPCD and the OPPS found that verbal and memory function in young children is poor when they have been exposed to cannabis during pregnancy.
The most consistent findings relate to impulsivity and hyperactivity. At 6 and 10 years of age, both the OPPS and MHPCD reported an increase in these behaviors, which was similarly found by the Generation R study who reported that at this age children were more like to exhibit aggressive, rule-breaking behavior than their peers. These behavioral changes don't stop during childhood, either: the children of mothers who used cannabis during pregnancy are more likely to abuse drugs such as opiates, tobacco and cannabis than their peers, and start this use earlier in life. Abnormal brain function associated with poor working memory are also found when these offspring reach adulthood. Thus, the data acquired from epidemiological studies of humans exposed to cannabis during pregnancy have revealed significant and lasting deficits which begin at birth and persist into adulthood.
Animal models
Laboratory research, using animal models of cannabis use during pregnancy and breastfeeding, has supported and expanded on these findings. Unlike with human research, however, animal models can provide insight into how the behavioral deficits caused by cannabis exposure correlate with specific changes in brain structure and function. As with humans, social and emotional behavior in early life is disturbed by cannabis exposure during pregnancy14-15. These disruptions are caused by changes in a region of the brain known as the prefrontal cortex, which is responsible for many so-called "executive functions" such as critical thinking and planning. These social deficits also extend into adolescence and adulthood.
Another strong correlation between observations in humans exposed to cannabis during gestation and animal models is propensity towards drug abuse. As above, unlike in human research, the specific brain circuitry and molecular mechanisms underlying these changes have been discovered in animal models. When rats are exposed to prenatal cannabis, they are more likely to develop a rapid addiction to opioids such as morphine16, especially if they are exposed to other stressful circumstances. This is likely due to changes in the brain's dopamine and opioid receptors which results from this cannabis exposure. Given the current opioid-epidemic in the United States, such findings paint a grim picture for children exposed to cannabis during pregnancy. These cannabis-exposed mouse offspring are also more sensitive to other drugs, like cannabis itself, later in their lives. Because rodents are not subject to the influences of such other factors as socioeconomic status, questionable maternal care, or environmental influences which may lead humans to drug abuse, the findings in rodents point to very fundamental changes in the brain's reward systems which cause an increase in the propensity towards drug abuse and addiction. Combined with these types of other factors, which may exacerbate these biological changes in humans, the risk for later-life drug abuse in humans exposed to cannabis during pregnancy may be more significant than that seen in rodents.
Finally, though few studies have thus far investigated the effects of cannabis exposure via breast milk, the data which have thus far been published paint a similar picture to that seen with exposure during pregnancy. Briefly, it is known that cannabinoids like THC and CBD are effectively transferred through breast milk and arrive in the brains and bodies of the milk-consuming offspring. Because these cannabinoids are highly lipophilic (meaning that they dissolve well in fats), and milk is a lipid-based liquid, the concentrations of THC and CBD in breast milk may actually be higher than that seen in the blood of the mother consuming the drug.
Our lab has investigated this by treating lactating rats with cannabinoids during the first 10 days of postnatal life, when rat pups are feeding exclusively on breast milk. We have shown that this cannabinoid exposure via breast milk causes significant delays in brain development in such crucial regions as the prefrontal cortex17, a hub of activity in the brain which is responsible for such important functions as planning, critical thinking, and other so-called "executive functions." We found that this delayed maturation of the prefrontal cortex is also correlated with retarded development of social communication in these young rodents. Our ongoing research is investigating the long-term consequences of these changes, which thus far appear to be persistent into adulthood. Thus, like cannabis exposure during pregnancy, cannabis use during breastfeeding is likely to cause significant, lasting changes in the brains of the breast-fed offspring.
Conclusions
Together, the findings in both human epidemiological studies and in laboratory modeling of cannabis exposure during pregnancy and breastfeeding highlight a number of important, lasting consequences. Even within the womb, brain region connectivity may be altered in the developing fetus by cannabis exposure. While physical deformities are not found in the offspring of cannabis using mothers, low birth weights are more common than amongst the general population. During very early development, cannabis-exposed children exhibit deficits in learning and social behavior, and are more likely to experience depressive and anxiety-like disorders, which also correlate with more "acting out," also known as externalizing behavior or aggression. These deficits in learning and social behavior continue through adolescence, where cannabis-exposed children are also more likely to initiate the use of addictive and recreational drugs such as tobacco, cannabis and even opiates like morphine. Finally, though current data are sparse, it appears that this array of deficits persists into adulthood, where the adult offspring of cannabis-using mothers are more likely to experience cognitive difficulties, social dysfunction, and a higher propensity towards drug abuse.
As of today, there are no known strategies or cures to ameliorate these lasting symptoms in cannabis-exposed offspring. While so-called "rescue strategies" have been demonstrated in some laboratory models, they remain theoretical and a distant cry from public application. The only sure-fire way of preventing these problems is by not consuming cannabis or cannabis-containing products during pregnancy or breastfeeding. Thus, while cannabis use in the general population may be increasingly accepted and considered to carry little or no significant risk, the use of cannabis by pregnant and breastfeeding women should be avoided. Obstetricians and gynecologists, as well as industry representatives for cannabis sales and distribution must acknowledge these dangers and work to ensure that access to cannabis comes with equal access to education.
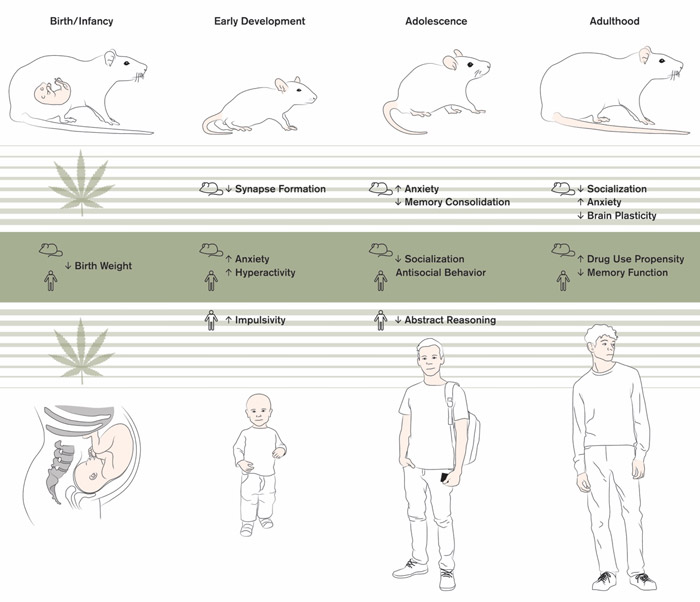
Figure 1. A lifetime of outcomes following cannabis exposure during pregnancy. From left to right, selective outcome measures from birth through adulthood in rodent studies (top), human studies (bottom), and findings which have been reproduced in both humans and rodents (middle). Adapted from Laura F Scheyer, Trends in Neurosciences, Scheyer et al., 2019.
References
1. Ko, J. Y., Farr, S. L., Tong, V. T., Creanga, A. A., & Callaghan, W. M. (2015). Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. American Journal of Obstetrics and Gynecology, 213(2), 201.e1-201.e10.
2. Dickson, B., Mansfield, C., Guiahi, M., Allshouse, A. A., Borgelt, L. M., Sheeder, J., ... Metz, T. D. (2018). Recommendations From Cannabis Dispensaries About First-Trimester Cannabis Use. Obstetrics and Gynecology, 131(6), 1031–1038.
3. Fried, P.A. and Watkinson, B. (2000) Visuoperceptual functioning differs in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 22, 11–20 70.
4. Fried, P.A. et al. (1992) A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol. Teratol. 14, 299–311.
5. Fried, P.A. (2002) Adolescents prenatally exposed to marijuana: examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J. Clin. Pharmacol. 42, 97S–102S.
6. Fried, P.A. et al. (2003) Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 25, 427–436.
7. Day, N. et al. (1992) The effects of prenatal tobacco and marijuana use on offspring growth from birth through 3 years of age. Neurotoxicol. Teratol. 14, 407–414.
8. Day, N.L. et al. (1994) Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol. Teratol. 16, 169–175.
9. Goldschmidt, L. et al. (2000) Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol. Teratol. 22, 325–336.
10. Goldschmidt, L. et al. (2004) Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol. Teratol. 26, 521–532.
11. Goldschmidt, L. et al. (2008) Prenatal marijuana exposure and intelligence test performance at age 6. J. Am. Acad. Child Adolesc. Psychiatry 47, 254–263. Goldschmidt, L. et al. (2012) School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol. Teratol. 34, 161–167.
12. El Marroun, H. et al. (2008) Demographic, emotional and social determinants of cannabis use in early pregnancy: the Generation R study. Drug Alcohol Depend. 98, 218–226.
13. Hofman, A. et al. (2004) Growth, development and health from early fetal life until young adulthood: the Generation R Study. Paediatr. Perinat. Epidemiol. 18, 61–72.
14. Trezza, V. et al. (2012) Altering endocannabinoid neurotransmission at critical developmental ages: impact on rodent emotionality and cognitive performance. Front. Behav. Neurosci. 6, 2.
15. Antonelli, T. et al. (2005) Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex 15, 2013–2020.
16. Vela, G. et al. (1998) Maternal exposure to delta9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 807, 101–109.
17. Scheyer, A.F. et al., Cannabinoid exposure via lactation in rats disrupts perinatal programming of the GABA trajectory and select early-life behaviors. Biol. Psychiatry Published online September 5, 2019.


